
Microbiology with Diseases by Body System (4th Edition)
4th Edition
ISBN: 9780321918550
Author: Robert W. Bauman Ph.D.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2VI
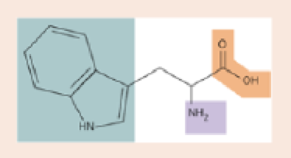
Shown is the amino acid tryptophan. Put the letter “C” at the site of every carbon atom. Label the amino group, the carboxyl group, and the side group.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Preoperative Diagnosis: Torn medial meniscus, left knee
Postoperative Diagnosis: Combination horizontal cleavage tear/flap tear, posterior horn, medial meniscus, left knee.
Operation: Arthroscopic subtotal medial meniscectomy, left knee
Anesthetic: General endotracheal
Description of Procedure: The patient was placed on the operating table in the supine position and general endotracheal anesthesia was administered. After an adequate level of anesthesia was achieved, the patient's left lower extremity was prepped with Betadine scrubbing solution, then draped in a sterile manner. Several sites were then infiltrated with 1% Xylocaine solution with Epinephrine to help control bleeding from stab wounds to be made at these sites. These stab wounds were made anterolaterally at the level of the superior pole of the patella for insertion of an irrigation catheter into the suprapatellar pouch area, anterolaterally at the level of the joint line for insertion of the scope and anteromedially at…
UARDIAN
SIGNA
Life Sciences/ Baseline Test
Grade 10
ry must be written in point form.
pot
in full sentences using NO MORE than 70 words
sentences from 1 to 7.
only ONE point per sentence.
words as far as possible.
number of words you have used in brackets at the end
GDE/2024
QUESTION 3
The table below shows the results of an investigation in which the effect of
temperature and light on the yield of tomatoes in two greenhouses on a farm
was investigated.
TEMPERATURE
(°C)
AVERAGE YIELD OF TOMATOES PER
3.1
PLANT
(kg)
LOW LIGHT LEVELS
HIGH LIGHT LEVELS
5
0,5
0,5
10
1,5
2,5
15
3,0
5,0
20
3,6
8,5
25
3,5
7,8
30
2,5
6,2
State TWO steps the investigator may have taken into
consideration during the planning stage of the investigation.
(2)
3.2
Identify the:
a) Independent variables
(2)
b) Dependent variable
(1)
3.3
Plot a line graph showing the results of the average yield of the
tomatoes from 5°C to 30°C for low light levels.
(6)
3.4
State ONE way in which the scientists could have improved the…
Explain why you chose this mutation. Begin by transcribing and translating BOTH the
normal and abnormal DNA sequences. The genetic code below is for your reference.
SECOND BASE OF CODON
כ
FIRST BASE OF CODON
O
THIRD BASE OF CODON
SCAGUCAGUGAGUCAG
UUU
UUC
UCU
UAU
UGU
Phenylalanine
(F)
Tyrosine (Y)
Cysteine (C)
UCC
UAC
UGC
Serine (S)
UUA
UUG
Leucine (L)
UCA
UCG_
UAA
UGA
Stop codon
-Stop codon
UAG
UGG -Tryptophan (W)
CUU
CUC
CCU
CAU
CGU
Histidine (H)
CCC
CAC
CGC
-Leucine (L)
Proline (P)
CUA
CCA
CAA
CUG
CCG
CAG-Glutamine (Q)
-Arginine (R)
CGA
CGG
AUU
ACU
AAU
AGU
AUC
Isoleucine (1)
Asparagine (N)
ACC
AAC
Threonine (T)
AUA
ACA
AAA
Methionine (M)
Lysine (K)
AUG
ACG
Start codon
AAG
AGC-Serine (S)
-Arginine (R)
AGA
AGG
GUU
GCU
GAU
GUC
GUA
GUG
GCC
Valine (V)
-Alanine (A)
GCA
GCG
GAC
GAA
GAG
Aspartic acid
(D)
GGU
Glutamic acid
(E)
GGC
GGA
GGG
Glycine (G)
In order to provide a complete answer to the question stated above, fill in the mRNA bases
and amino acid sequences by using the Genetic Code…
Chapter 2 Solutions
Microbiology with Diseases by Body System (4th Edition)
Ch. 2 - Electrons zip around the nucleus at about 5...Ch. 2 - Chlorine and potassium atoms form ionic bonds,...Ch. 2 - Why are decomposition reactions exothermic, that...Ch. 2 - Why does the neutralization of an acid by a base...Ch. 2 - Raw Oysters and Antacids: A Deadly Mix? The highly...Ch. 2 - Why do the cell membranes of microbes living in...Ch. 2 - Prob. 1MCCh. 2 - The atomic mass of an atom most closely...Ch. 2 - One isotope of iodine differs from another in...Ch. 2 - Prob. 4MC
Ch. 2 - Which of the following terms most correctly...Ch. 2 - In water, cations and anions of salts dissociate...Ch. 2 - Prob. 7MCCh. 2 - Which of the following statements about a...Ch. 2 - Proteins are polymers of ___________. a. amino...Ch. 2 - Which of the following are hydrophobic organic...Ch. 2 - Fill in the Blanks 1. The outermost electron shell...Ch. 2 - Fill in the Blanks 2. The type of chemical bond...Ch. 2 - Prob. 3FIBCh. 2 - Prob. 4FIBCh. 2 - Fill in the Blanks 5. Groups of atoms such as NH2...Ch. 2 - Fill in the Blanks 6. The reverse of dehydration...Ch. 2 - Fill in the Blanks 7. Reactions that release...Ch. 2 - Fill in the Blanks 8. All chemical reactions begin...Ch. 2 - Fill in the Blanks 9. The ____________ scale is a...Ch. 2 - Prob. 10FIBCh. 2 - Label a portion of the molecule below; label two...Ch. 2 - Shown is the amino acid tryptophan. Put the letter...Ch. 2 - List three main types of chemical bonds, and give...Ch. 2 - Name five properties of water that are vital to...Ch. 2 - Prob. 3SACh. 2 - What is the difference between atomic oxygen and...Ch. 2 - Explain how the polarity of water molecules makes...Ch. 2 - Prob. 1CTCh. 2 - Prob. 2CTCh. 2 - Two freshmen disagree about an aspect of...Ch. 2 - When an egg white is heated, it changes from...Ch. 2 - Prob. 5CTCh. 2 - The poison glands of many bees and wasps contain...Ch. 2 - Prob. 7CTCh. 2 - Prob. 8CTCh. 2 - The deadly poison hydrogen cyanide has the...Ch. 2 - Triple covalent bonds are stronger and more...Ch. 2 - How can hydrogen bonding between water molecules...Ch. 2 - How can a single molecule of magnesium hydroxide...Ch. 2 - Prob. 13CTCh. 2 - Prob. 14CTCh. 2 - A textbook states that only five nucleotide bases...Ch. 2 - Prob. 1CM
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- identify the indicated cell in white arrowarrow_forwardGloeocaspa Genus - diagram a colony and label the sheath, cell wall, and cytoplasm. Oscillatoria Genus - Diagram a trichome, and label the shealth and individual cells Nostoc Genus- diagram a sketch of the colonoy microscopically from low power to the left of the drawing. Draw a filament showing intercalary heterocysts, and vegatative cells to the right of the drawing Merismopedia Genus- diagram a sketch of the colony. draw and label a filament showing the colony, cell wall, and sheath. Gloeotrichia Genus- diagram a habit sketch of the colony. draw a filament showing the heterocyst, akimetes and vegatative cells of the filamentarrow_forwardOf this list shown, which genus does the image belong toarrow_forward
- As a medical professional, it is important to be able to discuss how genetic processes such as translation regulation can directly affect patients. Think about some situations that might involve translation regulation. Respond to the following in a minimum of 175 words: Why is translation regulation important? What are some examples of translation regulation in humans? Select one of the examples you provided and explain what happens when translation regulation goes wrong.arrow_forwardThe metabolic pathway below is used for the production of the purine nucleotides adenosine monophosphate (AMP) and guanosine monophosphate (GMP) in eukaryotic cells. Assume each arrow represents a reaction catalyzed by a different enzyme. Using the principles of feedback inhibition, propose a regulatory scheme for this pathway that ensures an adequate supply of both AMP and GMP, and prevents the buildup of Intermediates A through G when supplies of both AMP and GMP are adequate.arrow_forwardQUESTION 27 Label the structures marked A, B, C and explain the role of structure A. W plasma membrane For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS ☐ Paragraph Π " ΩΘΗ Β Open Sans, a... 10pt EEarrow_forward
- examples of synamptomorphyarrow_forwardexamples of synamtomorphy.arrow_forwardE. Bar Graph Use the same technique to upload the completed image. We will use a different type of graph to derive additional information from the CO2 data (Fig A1.6.2) 1. Calculate the average rate of increase in COz concentration per year for the time intervals 1959-1969, 1969- 1979, etc. and write the results in the spaces provided. The value for 1959-1969 is provided for you as an example. 2. Plot the results as a bar graph. The 1959-1969 is plotted for you. 3. Choose the graph that looks the most like yours A) E BAR GRAPH We will use a different type of graph to derive additional information from the CU, data (rig. nive). Average Yearly Rate of Observatory, Hawall interval Rate of increase per year 1959-1969 0.9 1969-1979 1979-1989 1989-1999 1999-2009 Figure A1.6.2 1999-2009 *- mrame -11- -n4 P2 جية 1989-1999 1979-1989 1969-1979 1959-1969 This bar drawn for you as an example 1.0 CO, Average Increase/Year (ppmv) B) E BAR GRAPH We will use a different type of graph to derive…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning


Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning


Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax


Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license