
Concept explainers
(a)
Interpretation:
Hybridization of underlined
Concept Introduction:
Hybridization is the hypothetical concept of mixing of atomic orbital into hybrid orbitals that are of dissimilar shapes, energies and are appropriate for combination of electrons to form bonds in
Hybridization is calculated by the hybrid orbitals and to calculate hybrid orbitals we need to know the steric number that is given by,
The table that relates the steric number with hybridization is as follows:
(a)
Answer to Problem 2F.8E
The molecule
Explanation of Solution
The molecule
The symbol for carbon is
The symbol for hydrogen is
Its Lewis structure is as follows:
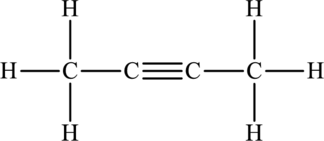
As from the structure above, 2 atoms are bonded to the underlined atom and 0 lone pair on it.
Substitute 2 for number of atoms bonded to the central atom and 0 for a number of lone pair on the central atom in equation (1) to find out the steric number.
Hence, the hybridization of
(b)
Interpretation:
Hybridization of underlined
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2F.8E
The molecule
Explanation of Solution
The molecule
The symbol for carbon is
The symbol for hydrogen is
The symbol for nitrogen is
Its Lewis structure is as follows:
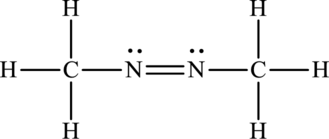
As from the structure above, 2 atoms are bonded to the underlined nitrogen atom and 1 lone pair on it.
Substitute 2 for a number of atoms bonded to the central atom and 1 for a number of lone pairs on the central atom in equation (1) to find out the steric number.
Hence, the hybridization of
(c)
Interpretation:
Hybridization of underlined
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2F.8E
The molecule
Explanation of Solution
The molecule
The symbol for carbon is
The symbol for hydrogen is
Its Lewis structure is as follows:
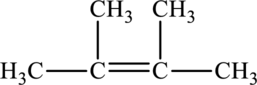
As from the structure above, 3 atoms are bonded to the underlined carbon atom and 0 lone pair on the same atom.
Substitute 3 for a number of atoms bonded to the central atom and 0 for a number of lone pair on the central atom in equation (1) to find out the steric number.
Hence, the hybridization of
(d)
Interpretation:
Hybridization of underlined
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2F.8E
The molecule
Explanation of Solution
The molecule
The symbol for carbon is
The symbol for hydrogen is
The symbol for nitrogen is
Its Lewis structure is as follows:
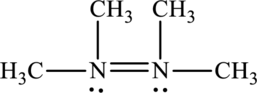
As from the structure above, 3 atoms are bonded to the underlined nitrogen atom and 1 lone pair on the same atom.
Substitute 3 for a number of atoms bonded to central atom and 1 for a number of lone pair on the central atom in equation (1) to find out the steric number.
Hence, the hybridization of
Want to see more full solutions like this?
Chapter 2 Solutions
CHEMICAL PRINCIPLES PKG W/SAPLING
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
- State the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
- For the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardR lactam or lactone considering as weak acid or weak base and whyarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





