
Concept explainers
(a)
Interpretation:
Dominant resonance hybrid structure among below two Lewis structures have to be determined.

Concept Introduction:
Resonance hybrid structures are set of two or more Lewis structures that illustrate electronic bonding through fractional bonds or fractional charges. Dominant resonance structure possesses the rules as follows:
1. Complete octet of each atom.
2. Smallest number of formal charges.
3. Most electronegative atom should possess negative charge and most electropositive atom should possess positive charge.
The formal charge is the charge assigned on each atom irrespective of its electronegativity so that electrons are equally shared between the atoms. The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Here,
(b)
Interpretation:
Dominant resonance hybrid structure among below two Lewis structures have to be determined.
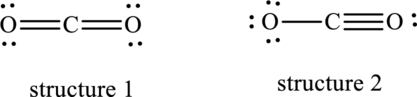
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
CHEMICAL PRINCIPLES PKG W/SAPLING
- The cyclohexane carboxylate anion has a Lewis structure Pushing a pair of unshared electrons away from the negatively charged oxygen atom and, at the same time, pushing a pair of pi electrons toward the other oxygen will generate a second resonance structure. Thus,arrow_forwardValine is an amino acid with this Lewis structure: Write the Lewis structure for the zwitterion form of valine.arrow_forwardComplete the following Lewis structures for the HONS molecule by adding dots for unshared valence electrons and indicating formal charges. Evaluate the importance of each structure as a contributor to a resonance hybrid. H – 0 – N = S, H–0 = N – Sarrow_forward
- Write the Lewis structure for the diatomic molecule P2, an unstable form of phosphorus found in hightemperature phosphorus vapor.arrow_forwardMany free radicals combine to form molecules that do not contain any unpaired electrons. The driving force for the radical-radical combination reaction is the formation of a new electron-pair bond. Consider the chemical equation. N(g)+NO(g) NNO(g) Write Lewis formulas for the reactant and product species in the chemical equation. Include nonbonding electrons.arrow_forwardA 0.167-g sample of an unknown compound contains 0.00278 mol of the compound. Elemental analysis of the compound gives the following percentages by mass: 40.00% C; 6.71% H; 53.29% O. Determine the molecular formula, molar mass, and Lewis structure of the unknown compound.arrow_forward
- A 0.167-g sample of an unknown acid requires 27.8 mL of 0.100 M NaOH to titrate to the equivalence point. Elemental analysis of the acid gives the following percentages by mass: 40.00% C, 6.71% H, 53.29% O. Determine the molecular formula, molar mass, and Lewis structure of the unknown acid.arrow_forwardDraw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- : a) The leftmost bond (between N and N) is a single bond.b) The rightmost bond (between N and N) is a single bond.c) The formal charge on the leftmost (N) atom is -1.d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4.e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.arrow_forwardDraw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- : a) The leftmost bond (between N and N) is a single bond. b) The rightmost bond (between N and N) is a single bond. c) The formal charge on the leftmost (N) atom is -1. d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4. e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.arrow_forward
- Describe the difference between a full Lewis structure and bond-line notation. What changes? Why is it easier to write?Dontarrow_forwardDescribe the Lewis Structure for an Ion with More Than an Octet of Electrons?arrow_forwardcarbon tetrachloride was formerly used in fire extinguishers for electrical fires.it is no longer used for this purpose because of the formation of the toxic gas phosgene,CL2CO.wRITE THE LEWIS STRUCTURES for the methane and ethane molecules.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



