
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.55P
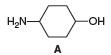
a. What is the conjugate acid of A?
b. What is the conjugate base of A?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw a tetramer of this alternating copolymer.
H
I
T
H
HH
H
-H
C.
H-
Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above.
H-
H
CIH
H
H
H
HHHH
H
H
0
·H
H–
冊
CH
CHI
HH C-
H-
H
H-
H
H
A.
H
H C
H
H-
-H
HH
H
B.
H-
-H
D.
H
H H
H
•
H
-H
E.
-H
H
H
HICH
T
HHH
F.
H-
Polylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.
Chapter 2 Solutions
PKG ORGANIC CHEMISTRY
Ch. 2 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2 - a. Draw the conjugate acid of each base:...Ch. 2 - Label each statement as True or False.
a. is the...Ch. 2 - Label the acid and base, and the conjugate acid...Ch. 2 - Decide which compound is the acid and which is the...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Use a calculator when necessary to answer the...Ch. 2 - Rank the conjugate bases of each of group of acids...Ch. 2 - Problem-2.10 Considers two acids: (formic acid,)...
Ch. 2 - Prob. 2.11PCh. 2 - Draw the products of each reaction and determine...Ch. 2 - Prob. 2.13PCh. 2 - Without reference to a pKa table, decide which...Ch. 2 - Rank the labeled H atoms in the following compound...Ch. 2 - Which hydrogen in each molecule is most...Ch. 2 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2 - Which compound in each pair of isomers is the...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2 - Explain the apparent paradox. HBr is a stronger...Ch. 2 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2 - Acetonitrile (CH3CN) has a pKa of 25, making it...Ch. 2 - For each pair of compounds: [1] Which indicated H...Ch. 2 - Rank the compounds in each group in order of...Ch. 2 - Which proton in each of the following drugs is...Ch. 2 - Which anion A or B is the stronger base? ABCh. 2 - Prob. 2.28PCh. 2 - Problem 2.29
Compounds like amphetamine that...Ch. 2 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2 - Which species are Lewis acids?
a. b. c. d.
Ch. 2 - For each reaction, label the Lewis acid and base....Ch. 2 - Prob. 2.33PCh. 2 - Prob. 2.34PCh. 2 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - 2.40 Draw the products formed from the acid-base...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 2.43PCh. 2 - Prob. 2.44PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Which compounds can be deprotonated by OH, so that...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - Rank the following compounds in order of...Ch. 2 - 2.51 Rank the following ions in order of...Ch. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - 2.54 The of three bonds is given below.
a. For...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - 2.56 Draw the structure of a constitutional isomer...Ch. 2 - 2.57 Many drugs are Bronsted-Lowry acids or...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 2.62PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 2.67PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 2.69PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - 2.72 DBU, is a base we will encounter in...Ch. 2 - 2.73 Molecules like acetamide can be protonated...Ch. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - 2.76 Write a stepwise reaction sequence using...Ch. 2 - Prob. 2.77PCh. 2 - 2.78 Which compound, M or N, is the stronger acid?...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY