
Concept explainers
(a)
Interpretation:
The polarity of the given molecule is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
The given molecule A is nonpolar.
Explanation of Solution
![]()
The given molecule is in trans form. The directions of the vectors of both the C-F bonds are equal but opposite to each other. Hence the dipole moments of both the C-F bonds get cancelled out with each other. Therefore, there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule A is nonpolar.
(b)
Interpretation:
The polarity of the given molecule is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
The given molecule B is polar.
Explanation of Solution
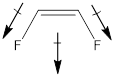
The given molecule is in cis form. The direction of vectors of both the C-F bonds is in the same direction, giving a net permanent dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule B is polar.
(c)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule C is nonpolar.
Explanation of Solution
![]()
In this molecule, both the C-Cl bonds are opposite to each other, so the dipole moments are cancelled out with each other. Therefore, there is no net dipole moment in this molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule C is nonpolar.
(d)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a.
Answer to Problem 2.41P
The given molecule D is polar.
Explanation of Solution
![]()
In this molecule, chlorine is more electronegative than the carbon atom; hence the direction of the vector of dipole moment is more towards C-Cl bond, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule D is polar.
(e)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule E is polar.
Explanation of Solution
![]()
In this molecule, Chlorine is more electronegative than bromine; hence the direction of the vector of dipole moment is more towards C-Cl bond, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule E is polar.
(f)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule F is nonpolar.
Explanation of Solution
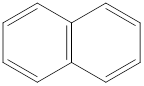
In this molecule, there is no electronegative atom present since no charge separation is taking place. So there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule F is nonpolar.
(g)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule G is polar.
Explanation of Solution
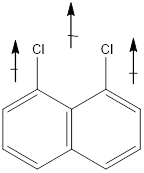
In this molecule, Chlorine is an electronegative atom, and both the C-Cl bonds are in the same direction. Therefore, the direction of the vector of dipole is moment is upward, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule G is polar.
(h)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule H is nonpolar.
Explanation of Solution
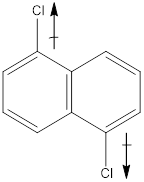
In the molecule, chlorine is an electronegative atom, and both the C-Cl bonds are in opposite direction. Therefore, the directions of the vectors of dipole moment of two C-Cl bonds get cancelled out with each other. Hence there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule H is nonpolar.
(i)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule I is polar.
Explanation of Solution
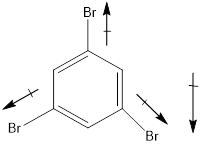
In this molecule, bromine is an electronegative atom, but one C-Br bond is in upward direction, and two C-Br bonds are in downward direction. Therefore, the net dipole moment acts in downward direction.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule I is polar.
(j)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule J is polar.
Explanation of Solution
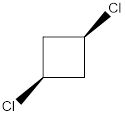
In this molecule, both the C-Cl bonds are present above the plane (that is wedge notation). Therefore, the directions of the vectors of dipole moment of both the C-Cl bonds are in the same direction, giving net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule J is polar.
(k)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule K is polar.
Explanation of Solution
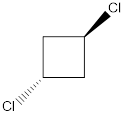
In this molecule, one C-Cl bond is present above the plane (that is, the wedge notation), and another C-Cl bond is present below the plane (that is, the dotted notation). Therefore, the directions of the vectors of dipole moment of both the C-Cl bonds are in opposite direction, which get cancelled out with each other, giving no net dipole moment to the molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule K is nonpolar.
(l)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule L is polar.
Explanation of Solution

In this molecule, though both the C-Cl bonds are in opposite direction, both the chlorines are present on carbon
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule L is polar.
(m)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule M is nonpolar.
Explanation of Solution
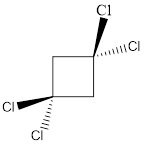
In this molecule, two C-Cl bonds are above the plane, and two C-Cl bonds are below the plane; hence the molecule has symmetry. The directions of the vectors of dipole moment of all the four C-Cl bonds are cancelled with each other, giving no net dipole moment to the molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule M is nonpolar.
(n)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule N is polar.
Explanation of Solution
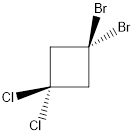
In this molecule, two C-Cl and two C-Br bonds are present. Since chlorine is more electronegative than bromine, the direction of the vector of dipole moment is towards C-Cl bonds. Therefore, there is a net dipole moment present in this molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule N is polar.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry: Principles And Mechanisms
- CUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardWhat does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forwardIdentifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- * Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



