
Chemistry (7th Edition)
7th Edition
ISBN: 9780321943170
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 2.40CP
Interpretation Introduction
To determine:
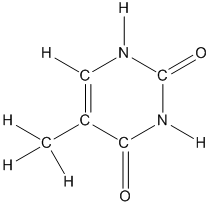
What would be the chemical formula of thymine from the given structure? To list the element symbols in alphabetical order and to give the number of each element in subscript.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How to draw this claisen condensation reaction mechanisms/
Write all of Me Possible Products For each
Of the Following reactions. In each case identity
all pains of enantiomers, all digsterzoners and
all Meso compounds
9.
11-60
11-0-11
V-G
Η
Η
H
~ C-11 +HB+ -
1
H
b.
पन्ना
171-0-11
H-C-H
Н
C-C=c-call +HBr Perendez
==
How can i draw the mechanisms for this molecule?
Chapter 2 Solutions
Chemistry (7th Edition)
Ch. 2 - Conceptual PRACTICE 2.1 An element is a shiny,...Ch. 2 - Prob. 2.2ACh. 2 - PRACTICE 2.3Compounds A and B are colorless gases...Ch. 2 - APPLY 2.4If the chemical formula of compound A in...Ch. 2 - PRACTICE 2.5The gold foil that Rutherford used in...Ch. 2 - Prob. 2.6ACh. 2 - PRACTICE 2.7The isotope 3475Se is used medically...Ch. 2 - APPLY 2.8Element X is toxic to humans in high...Ch. 2 - PRACTICE 2.9Copper metal has two naturally occur-...Ch. 2 - Prob. 2.10A
Ch. 2 - Prob. 2.11PCh. 2 - APPLY 2.12If 2.26 1022 atoms Of element Y have a...Ch. 2 - Prob. 2.13PCh. 2 - Prob. 2.14ACh. 2 - Prob. 2.15PCh. 2 - Prob. 2.16ACh. 2 - Prob. 2.17PCh. 2 - Prob. 2.18ACh. 2 - Prob. 2.19PCh. 2 - Prob. 2.20ACh. 2 - Prob. 2.21PCh. 2 - Prob. 2.22ACh. 2 - Prob. 2.23PCh. 2 - Prob. 2.24ACh. 2 - Prob. 2.25PCh. 2 - Prob. 2.26PCh. 2 - Prob. 2.27PCh. 2 - Prob. 2.28PCh. 2 - Prob. 2.29PCh. 2 - Prob. 2.30PCh. 2 - Prob. 2.31CPCh. 2 - Prob. 2.32CPCh. 2 - Prob. 2.33CPCh. 2 - Prob. 2.34CPCh. 2 - Prob. 2.35CPCh. 2 - Prob. 2.36CPCh. 2 - Prob. 2.37CPCh. 2 - Prob. 2.38CPCh. 2 - Prob. 2.39CPCh. 2 - Prob. 2.40CPCh. 2 - Prob. 2.41CPCh. 2 - Prob. 2.42CPCh. 2 - Prob. 2.43CPCh. 2 - Prob. 2.44SPCh. 2 - Prob. 2.45SPCh. 2 - Prob. 2.46SPCh. 2 - Prob. 2.47SPCh. 2 - Prob. 2.48SPCh. 2 - Prob. 2.49SPCh. 2 - Prob. 2.50SPCh. 2 - Prob. 2.51SPCh. 2 - Prob. 2.52SPCh. 2 - Prob. 2.53SPCh. 2 - Prob. 2.54SPCh. 2 - Prob. 2.55SPCh. 2 - Where in the periodic table are the metallic...Ch. 2 - Prob. 2.57SPCh. 2 - Prob. 2.58SPCh. 2 - Prob. 2.59SPCh. 2 - 2.60 List several general properties of the...Ch. 2 - Prob. 2.61SPCh. 2 - Prob. 2.62SPCh. 2 - Prob. 2.63SPCh. 2 - At room temperature, a certain element is found to...Ch. 2 - Prob. 2.65SPCh. 2 - At room temperature, a certain element is yellow...Ch. 2 - Prob. 2.67SPCh. 2 - Prob. 2.68SPCh. 2 - Prob. 2.69SPCh. 2 - How does Dalton’s atomic theory account for the...Ch. 2 - Prob. 2.71SPCh. 2 - A sample of mercury with a mass of 114.0 g was...Ch. 2 - Prob. 2.73SPCh. 2 - In methane, one part hydrogen combine with three...Ch. 2 - In borane, one part hydrogen combine with 3.6...Ch. 2 - Benzene, ethane, and ethylene are just three of a...Ch. 2 - Prob. 2.77SPCh. 2 - Prob. 2.78SPCh. 2 - 2.79 In addition to carbon monoxide (CO) and...Ch. 2 - Prob. 2.80SPCh. 2 - Prob. 2.81SPCh. 2 - Prob. 2.82SPCh. 2 - What affects the magnitude of the deflection of...Ch. 2 - Prob. 2.84SPCh. 2 - Prob. 2.85SPCh. 2 - Which of the following charges is NOT possible for...Ch. 2 - What discovery about atomic structure was made...Ch. 2 - Prior to Rutherford’s gold foil experiment, the...Ch. 2 - A period at the end of sentence written with a...Ch. 2 - A 1/4 inch thick lead sheet is used for protection...Ch. 2 - Prob. 2.91SPCh. 2 - What is the difference between an atom’s atomic...Ch. 2 - Prob. 2.93SPCh. 2 - Prob. 2.94SPCh. 2 - Prob. 2.95SPCh. 2 - Prob. 2.96SPCh. 2 - The radioactive isotope cesium-137 was produced in...Ch. 2 - Prob. 2.98SPCh. 2 - Prob. 2.99SPCh. 2 - How many protons, neutrons, and electrons are in...Ch. 2 - Prob. 2.101SPCh. 2 - Prob. 2.102SPCh. 2 - Prob. 2.103SPCh. 2 - Prob. 2.104SPCh. 2 - Prob. 2.105SPCh. 2 - Prob. 2.106SPCh. 2 - Prob. 2.107SPCh. 2 - Prob. 2.108SPCh. 2 - Naturally occurring silver consists of two...Ch. 2 - Prob. 2.110SPCh. 2 - Prob. 2.111SPCh. 2 - Prob. 2.112SPCh. 2 - Prob. 2.113SPCh. 2 - Prob. 2.114SPCh. 2 - Prob. 2.115SPCh. 2 - Prob. 2.116SPCh. 2 - Prob. 2.117SPCh. 2 - Prob. 2.118SPCh. 2 - Prob. 2.119SPCh. 2 - Prob. 2.120SPCh. 2 - Which of the following bonds are likely to be...Ch. 2 - The symbol CO stands for carbon monoxide, but the...Ch. 2 - Prob. 2.123SPCh. 2 - Prob. 2.124SPCh. 2 - Prob. 2.125SPCh. 2 - Prob. 2.126SPCh. 2 - Prob. 2.127SPCh. 2 - Prob. 2.128SPCh. 2 - Prob. 2.129SPCh. 2 - Prob. 2.130SPCh. 2 - Prob. 2.131SPCh. 2 - Give systematic names for the following binary...Ch. 2 - Give systematic names for the following binary...Ch. 2 - Prob. 2.134SPCh. 2 - Prob. 2.135SPCh. 2 - Prob. 2.136SPCh. 2 - Prob. 2.137SPCh. 2 - Give systematic names for the following compounds:...Ch. 2 - Name the following ions: (a) Ba2+ (b) Cs+ (c) V3+...Ch. 2 - Prob. 2.140SPCh. 2 - Prob. 2.141SPCh. 2 - Prob. 2.142SPCh. 2 - Prob. 2.143SPCh. 2 - Prob. 2.144SPCh. 2 - Prob. 2.145SPCh. 2 - Prob. 2.146SPCh. 2 - Prob. 2.147SPCh. 2 - Prob. 2.148SPCh. 2 - Prob. 2.149SPCh. 2 - Prob. 2.150SPCh. 2 - Prob. 2.151SPCh. 2 - Germanium has five naturally occurring isotopes:...Ch. 2 - Prob. 2.153CPCh. 2 - Ammonia (NH3) and hydrazine (N2H4) are both...Ch. 2 - Prob. 2.155CPCh. 2 - Prob. 2.156CPCh. 2 - Prob. 2.157CPCh. 2 - Prob. 2.158CPCh. 2 - What was the mass in atomic mass units of a 40Ca...Ch. 2 - Prob. 2.160CPCh. 2 - Prob. 2.161CPCh. 2 - Prob. 2.162CPCh. 2 - Prob. 2.163CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forwardhelp draw any straightchain moleculearrow_forward
- How to do the mechanism drawn for the reactionarrow_forwardPlease provide the mechanism for this reacitonarrow_forwardQuestion 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forward
- Question 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forwardA. Add Water to below compound which 2-methyl 2-butene (addition Reaction) H₂C CH₂ CH, + H₂O-> ? Major product? Minor product? B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + Br₂→ ? Major product and Minor product both are same in this? C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition Reaction) H,C CH₂ CH₂ + HBr Major product? Minor product? D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + H₂ Major product and Minor product both are same in this?arrow_forward36) Complete the following multi-step reactions showing applications of enolate ions arising from ketones, esters, malonic ester, and keto ester, etc. (30 pts) (1) A NaOH, H₂O+ heat A NaOEt EtO OEt (11) EOH, H+ H. B LDA, H₂O+ -78°C B (i) NaOMe, Et-Br (ii) H₂O+, heat EtOOC (III) COOEt B A (i) NaOEt LiAlH 4-bromo-2-butene H₂O+ (ii) H3O+, heat Write the mechanism for Aldol Condensation (I A or B), and Claisen Condensation (II A).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY