
(a)
Interpretation:
The plot of ln y versus x on rectangular coordinates passes through (1.0, 693) and (2,0) should be drawn.
Concept introduction:
The straight-line plot has following equation:
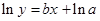
Here, 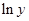 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 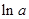 is intercept.
is intercept.
And, the following equation shows the exponential plot:
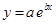
(b)
Interpretation:
The semilog plot of y versus x passes through (1,2) and (2,1) should be drawn.
Concept introduction:
The straight-line plot has following equation:
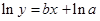
Here, 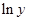 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 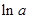 is intercept.
is intercept.
And, the following equation shows the exponential plot:
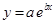
(c)
Interpretation:
A log pot y versus x passes through (1,2) and (2,1), determine the equation.
Concept introduction:
The straight-line plot has following equation:
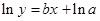
Here, 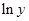 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 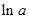 is intercept.
is intercept.
And, the following equation shows the exponential plot:
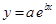
(d)
Interpretation:
A semilog plot of xy (logarithmic axis) versus y/x passes through (1.0. 40.2). Determine the equation.
Concept introduction:
The straight-line plot has following equation:
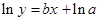
Here, 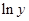 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 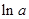 is intercept.
is intercept.
And, the following equation shows the exponential plot:
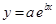
(e)
Interpretation:
The equation of log plot of y2/x versus (x-2) passes through (1.0, 40.2) and (2.0, 807.0)
Concept introduction:
The straight-line plot has following equation:
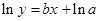
Here, 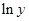 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 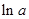 is intercept.
is intercept.
And, the following equation shows the exponential plot:
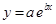
Trending nowThis is a popular solution!

Chapter 2 Solutions
Elementary Principles of Chemical Processes 4e Binder Ready Version + WileyPLUS Registration Card (Wiley Plus Products)
- Derive the 3D density of states for a system of electrons with the dispersion relation (in the picture) Assume that there is no spin degeneracyarrow_forwardQ2) Design a Full Adder to add two numbers each of 3 digits.arrow_forwardQ1) Design a Multiplexing system (shown below) to transmit six channels each of 10Kbps speed, through single channel with the following channel sequence: ch6, then ch1, then ch5, then ch2, then ch4, then ch3. The available clock is of 120KHz. (hint use jk flip flop in the timing and coding unit). Timing & code generator for the Clock 120KHz requested sequence CO C1 $2 --- $1 38 8 C2 Multiplexer C3 C4 C5arrow_forward
- Q3) Compare two numbers (A,B) each of two digits either A>b or Aarrow_forwardHow will you select a suitable condensate techniquearrow_forwardA biofilm consists of living cells immobilized in a gelatinous matrix. A toxic organic solute (species A) diffuses into the biofilm and is degraded to harmless products by the cells within the biofilm. We want to treat 0.2 m3 per hour of wastewater containing 0.14 mole/m of the toxic substance phenol using a system consisting of biofilms on rotating disk as shown below. thickness of the biofilm is 1 mm and we are interested to reduce the phenol concentration in the outlet stream to 0.025 mole/m3. The rate of disappearance of phenol (species A) within the biofilm is described by the following equation rA = - KICA Where k1 = 0.0024 s-1 The diffusivity of phenol in the biofilm at the process temperature of 25°C is 2.0x10 10 m2/s. Phenol is equally soluble in both water and the biofilm. 1. The concentration of A in the biofilm is give by Ca = Bisinh(mz) + Bzcosh(mz), the numerical value of m is. 2. Use m = 3 mm^-1, determine the numerical values with units for B1 and B2 with the unit of…arrow_forwarduse the integral method if you can, thank you!arrow_forward2. A single-effect evaporator is concentrating a feed solution of organic colloids from 5 to 50 wt %. The solution has a negligible boiling point elevation. The heat capacity of the feed is cp . 4.06 kJ/kg K and the feed enters at 15.6 °C. Saturated steam at 101.32 kPa is available for heating, and the pressure in the vapor space of the evaporator is 15.3 kPa. A total of 4536 kg/h of water is to be evaporated. The overall heat-transfer coefficient is 1988 W/m². K. What is the required surface area in m² and the steam consumption?arrow_forwardA mixture with 4% n-pentane, 40% n-hexane, 50% n-heptane, and 6% n-octane is to be distilled at 14.7 lb/in² (1 atm.) with 98% of the hexane and 1% of the heptane recovered in the distillate. If the liquid feed is a saturated liquid (i.e., q = 1). Calculate i.) The product compositions, ii.) The top and bottom temperature iii.) Minimum reflux ratio (Rm), iv.) Minimum theoretical plates and v.) Actual plates when the reflux ratio is 2Rm. Table 1: Component boiling point and molar mass Component Boiling point n-C5 97°F n-C6 156.2°F n-C7 209.1°F n-C8 258.1°F Molar mass 72.2 86.2 100.2 114.2 Hint: Since 1% of heptane is specified to be recovered in the distillate, 99% of heptane is to be recovered in the bottom.arrow_forwardH.W 1. A plant wishes to dry a certain type of fiberboard. To determine drying characteristics, a sample of 0.3 × 0.3 m with edges sealed was suspended from a balance and exposed to a current of hot dry air. The initial moisture content was 75%. The sheet lost weight at the rate of 1 104 Kg/s unit the moisture content fell to 60%. It was established that the equilibrium moisture content was 10 %. The dry mass of the sample was 0.90 Kg. All moisture contents were on a wet basis. Determine the time for drying the sheets from 75% to 20% moisture under the same drying conditions?arrow_forward2.) A mixture with 4% n-pentane, 40% n-hexane, 50% n-heptane, and 6% n-octane is to be distilled at 14.7 lb/in² with 98% of the hexane and 1% of the heptane recovered in the distillate. If the feed is a saturated liquid (q = 1), the top and bottom temperature are 149°F and 212°F respectively, calculate i.) The product compositions, ii.) Minimum reflux ratio (Rm), iii.) Minimum theoretical plates and iv.) Actual plate when the reflux ratio is 2Rm.arrow_forwardanswer choices 3. A. 0.18, B. 0.44, C. 0.01, D. 2 4. A. 0.2, B. 01, C. 0.4, D. 0.065 5.A. 1.43, B. 5.72, C. 0.93, D. 2.86 6. A. 1.0, B. 4.0, . 2.0, D. 0.65 7.A. 15.5, B. 8.0, C. 11.0, D. 58.5arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





