
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.36P
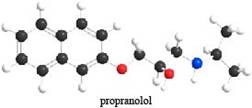
Propranolol is an antihypertensive agent—that is, it lowers blood pressure. (a)
Which proton in propranolol is most acidic? (b) What products are formed when propranolol is treated with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at
4.1 ppm? Select the single best answer.
The
H
O
HỌC—C—0—CH, CH,
2
A
ethyl acetate
H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm
Check
OA
B
OC
ch
B
C
Save For Later
Submit Ass
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
How many signals do you expect in the H NMR spectrum for this molecule?
Br Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red
Note for advanced students: In this question, any multiplet is counted as one signal.
1
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
O
✓
No additional Hs to color in top
molecule
ง
No additional Hs to color in bottom…
in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstant
Chapter 2 Solutions
ORGANIC CHEMISTRY
Ch. 2 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2 - a. Draw the conjugate acid of each base:...Ch. 2 - Label each statement as True or False.
a. is the...Ch. 2 - Label the acid and base, and the conjugate acid...Ch. 2 - Decide which compound is the acid and which is the...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Use a calculator when necessary to answer the...Ch. 2 - Rank the conjugate bases of each of group of acids...Ch. 2 - Problem-2.10 Considers two acids: (formic acid,)...
Ch. 2 - Prob. 2.11PCh. 2 - Draw the products of each reaction and determine...Ch. 2 - Prob. 2.13PCh. 2 - Without reference to a pKa table, decide which...Ch. 2 - Rank the labeled H atoms in the following compound...Ch. 2 - Which hydrogen in each molecule is most...Ch. 2 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2 - Which compound in each pair of isomers is the...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2 - Explain the apparent paradox. HBr is a stronger...Ch. 2 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2 - Acetonitrile (CH3CN) has a pKa of 25, making it...Ch. 2 - For each pair of compounds: [1] Which indicated H...Ch. 2 - Rank the compounds in each group in order of...Ch. 2 - Which proton in each of the following drugs is...Ch. 2 - Which anion A or B is the stronger base? ABCh. 2 - Prob. 2.28PCh. 2 - Problem 2.29
Compounds like amphetamine that...Ch. 2 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2 - Which species are Lewis acids?
a. b. c. d.
Ch. 2 - For each reaction, label the Lewis acid and base....Ch. 2 - Prob. 2.33PCh. 2 - Prob. 2.34PCh. 2 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - 2.40 Draw the products formed from the acid-base...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 2.43PCh. 2 - Prob. 2.44PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Which compounds can be deprotonated by OH, so that...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - Rank the following compounds in order of...Ch. 2 - 2.51 Rank the following ions in order of...Ch. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - 2.54 The of three bonds is given below.
a. For...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - 2.56 Draw the structure of a constitutional isomer...Ch. 2 - 2.57 Many drugs are Bronsted-Lowry acids or...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 2.62PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 2.67PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 2.69PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - 2.72 DBU, is a base we will encounter in...Ch. 2 - 2.73 Molecules like acetamide can be protonated...Ch. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - 2.76 Write a stepwise reaction sequence using...Ch. 2 - Prob. 2.77PCh. 2 - 2.78 Which compound, M or N, is the stronger acid?...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY