
Concept explainers
(a)
Interpretation:
The isomeric structures of octane having five carbons in their principal chains are to be drawn. The naming for all the structures is to be stated.
Concept introduction:
The naming of the chemical compound is done using the parameters given by IUPAC. IUPAC stands for International Union of Pure and Applied Chemistry. This system of nomenclature is accepted worldwide. The IUPAC system provides the set of rules in order to do the naming of the chemical compounds.
Isomers are the chemical compounds which have same chemical formula but different arrangement of molecules.
Answer to Problem 2.26AP
The isomeric structures of octane having five carbons in their principal chains along with their names are drawn below as,
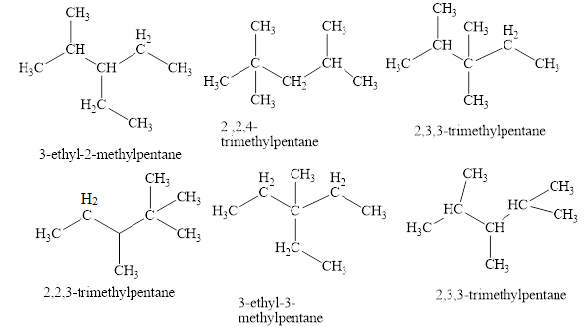
Explanation of Solution
The structure of octane can be drawn as shown in figure 1.
![]()
Figure 1
The isomers of the octane having five carbons in their principal chains can be drawn by rearranging the carbon atoms.
The first isomer is shown in figure 2 as,
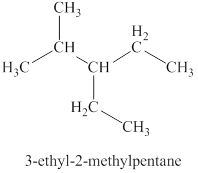
Figure 2
For the naming of the compound, the longest carbon chain is selected first. In the given compound the longest carbon chain has
The second isomer is shown in figure 3 as,
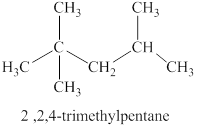
Figure 3
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The third isomer is shown in figure 4 as,
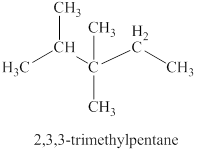
Figure 4
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The fourth isomer is shown in figure 5 as,
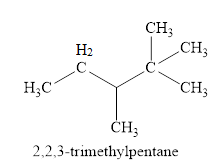
Figure 5
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The fifth isomer is shown in figure 6 as,
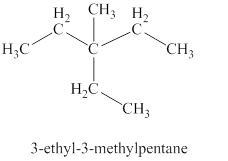
Figure 6
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The sixth isomer is shown in figure 7 as,
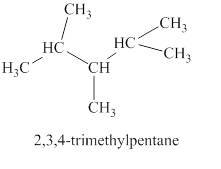
Figure 7
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The isomeric structures of octane having five carbons in their principal chains along with their names are shown in figure 2 to 7.
(b)
Interpretation:
The isomeric structures of octane having six carbons in their principal chains are to be drawn. The naming for all the structures is to be stated.
Concept introduction:
The naming of the chemical compound is done using the parameters given by IUPAC. IUPAC stands for International Union of Pure and Applied Chemistry. This system of nomenclature is accepted worldwide. The IUPAC system provides the set of rules in order to do the naming of the chemical compounds.
Isomers are the chemical compounds which have same chemical formula but different arrangement of molecules.
Answer to Problem 2.26AP
The isomeric structures of octane having six carbons in their principal chains along with their names are drawn below as,
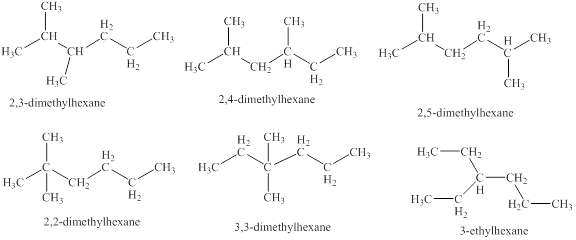
Explanation of Solution
The isomers of the octane having six carbons in their principal chains can be drawn by rearranging the carbon atoms.
The first isomer is shown in figure 8 as,
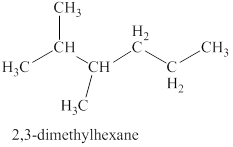
Figure 8
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The second isomer is shown in figure 9 as,
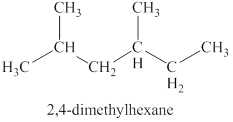
Figure 9
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The third isomer is shown in figure 10 as,
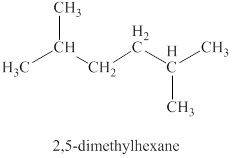
Figure 10
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The fourth isomer is shown in figure 11 as,
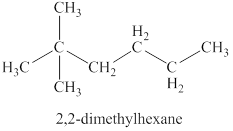
Figure 11
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The fifth isomer is shown in figure 12 as,
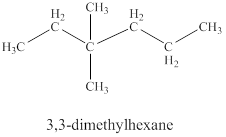
Figure 12
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest carbon chain has
The sixth isomer is shown in figure 13 as,
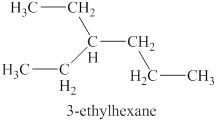
Figure 13
For the naming of the compound the longest carbon chain is selected first. In the given compound the longest chain carbon has
The isomeric structures of octane having six carbons in their principal chains along with their names are shown in figure 8 to 13.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





