
Concept explainers
Draw a “formula” for each of the following molecules using circular symbols of your choice to represent atoms:
a. A diatomic molecule of an element
b. A diatomic molecule of a compound
c. A triatomic molecule of an element
d. A molecule of a compound containing one atom of one element and four atoms of another element
(a)
Interpretation:
The formula for the diatomic molecule of an element by using circular symbols to represent atoms is to be drawn.
Concept introduction:
A molecular formula represents the number of atoms of each element present in a molecule of a compound.
The number of atoms present in molecule is determined by the subscript written below the normal line in the molecular formula.
Answer to Problem 2.1E
The formula for the diatomic molecule of an element by using circular symbols to represent atoms is shown below.
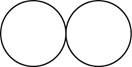
Explanation of Solution
It is given that the molecule of an element is a diatomic which means that the compound consists of two atoms with similar identity. This can be explained with the help of one example. Considering a diatomic molecule of an element that is chlorine gas. The chemical formula of chlorine gas is
Therefore, the formula for the diatomic molecule of an element by using circular symbols to represent atoms is shown below.

Figure 1
In the given figure, white circles represent the chlorine atoms.
The formula for the diatomic molecule of an element by using circular symbols to represent atoms is shown in figure 1.
(b)
Interpretation:
The formula for the diatomic molecule of a compound by using circular symbols to represent atoms is to be drawn.
Concept introduction:
A molecular formula represents the number of atoms of each element present in a molecule of a compound.
The number of atoms present in molecule is determined by the subscript written below the normal line in the molecular formula.
Answer to Problem 2.1E
The formula for the diatomic molecule of a compound by using circular symbols to represent atoms is shown below.
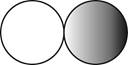
Explanation of Solution
It is given that the molecule of a compound is diatomic which means that the compound consists of two different atoms. This can be explained with the help of one example. Considering an example that is hydrogen fluoride. The chemical formula of hydrogen fluoride is
Therefore, the formula for the diatomic molecule of a compound by using circular symbols to represent atoms is shown below.
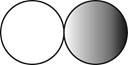
Figure 2
In the given figure, grey circle represent the fluorine atom, whereas white circle represent the hydrogen atoms.
The formula for the diatomic molecule of a compound by using circular symbols to represent atoms is shown in figure 2.
(c)
Interpretation:
The formula for the triatomic molecule of an element by using circular symbols to represent atoms is to be drawn.
Concept introduction:
A molecular formula represents the number of atoms of each element present in a molecule of a compound.
The number of atoms present in molecule is determined by the subscript written below the normal line in the molecular formula.
Answer to Problem 2.1E
The formula for the triatomic molecule of an element by using circular symbols to represent atoms is shown below.
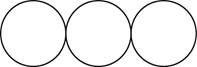
Explanation of Solution
It is given that the molecule of an element is a triatomic which means that the compound consists of three atoms with similar identity. This can be explained with the help of one example. Considering an example that is ozone. The chemical formula of ozone is
Therefore, the formula for the triatomic molecule of an element by using circular symbols to represent atoms is shown below.

Figure 3
In the given diagram, white circles represent oxygen atoms.
The formula for the triatomic molecule of an element by using circular symbols to represent atoms is shown in figure 3.
(d)
Interpretation:
The formula for a molecule of a compound containing one atom of one element and four atoms of another element by using circular symbols to represent atoms is to be drawn.
Concept introduction:
A molecular formula represents the number of atoms of each element present in a molecule of a compound.
The number of atoms present in molecule is determined by the subscript written below the normal line in the molecular formula.
Answer to Problem 2.1E
The formula for a molecule of a compound containing one atom of one element and four atoms of another element by using circular symbols to represent atoms is shown below.
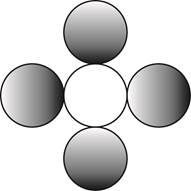
Explanation of Solution
It is given that the molecule contains one atom of one element and four atoms of another element. This can be explained with the help of one example. Considering an example that is carbon tetrachloride. The chemical formula of carbon tetrachloride is
Therefore, the formula for a molecule of a compound containing one atom of one element and four atoms of another element by using circular symbols to represent atoms is shown below.
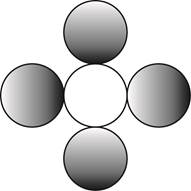
Figure 4
In the given diagram, white circle represent carbon atom, whereas grey circle represent chlorine atoms.
The formula for a molecule of a compound containing one atom of one element and four atoms of another element by using circular symbols to represent atoms is shown in figure 4.
Want to see more full solutions like this?
Chapter 2 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
Additional Science Textbook Solutions
Microbiology Fundamentals: A Clinical Approach
Campbell Essential Biology with Physiology (5th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Fundamentals Of Thermodynamics
Physical Science
Organic Chemistry
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor. одarrow_forwardQ9: Explain why compound I is protonated on O while compound II is protonated on N. NH2 DD I II NH2arrow_forwardComplete the following reaction by identifying the principle organic product of the reactionarrow_forward
- Denote the dipole for the indicated bonds in the following molecules. ✓ H3C CH3 B F-CCl3 Br-Cl H3C —Si(CH3)3 CH3 OH HO HO H HO OH vitamin Carrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward10:16 ☑ Vo)) Vo) 4G LTE 76% Complete the following reaction by identifying the principle organic product of the reaction. HO OH ↑ CH2N2 OH ? ○ A. 01 N₂H2C OH ОН B. HO OCH3 OH ○ C. HO OH ŎCH₂N2 ○ D. H3CO OH он Quiz navigation 1 2 3 4 5 11 12 Next page 10 6 7 8 9 10arrow_forward
- Which one of the following statements explain why protecting groups are referred to as “a necessary evil in organic synthesis”? Question 12Select one or more: A. They increase the length and cost of the synthesis B. Every synthesis employs protecting groups C. Protecting group have no role to play in a synthesis D. They minimize the formation of side productsarrow_forwardWhich of the following attributes is a key advantage of the chiral auxiliary approach over the chiral pool approach in asymmetric synthesis? Question 10Select one: A. Chiral auxiliaries are cheaper than chiral pool substrates B. Chiral auxiliary can be recovered and recycled unlike chiral pool substrates. C. The use of chiral auxiliaries provide enantiopure products, while chiral pool reactions are only enantioselective D. The chiral auxiliaries are naturally occurring and do not require synthesisarrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 CH3 H3C HO: CI:arrow_forward
- Which of the following are TRUE about linear syntheses? Question 7Select one: A. They are easier to execute B. They are the most efficient strategy for all syntheses C. They are generally shorter than convergent syntheses D. They are less versatile compared to convergent synthesesarrow_forwardWhich of the following characteristics is common among chiral pool substrates? Question 4Select one: A. They have good leaving groups B. They are all achiral C. All have a multiplicity of chiral centres D. They have poor leaving groupsarrow_forwardDetermine whether the following reaction is an example of a nucleophilic substitution reaction: H NO2 H+ NO 2 + Molecule A Molecule B Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. What word or two-word phrase is used to describe the role Molecule A plays in this reaction? What word or two-word phrase is used to describe the role Molecule B plays in this reaction? Use a 6 + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. O Yes ○ No ☐ 0 dx 000 HE ?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





