
(a)
Interpretation: The reagents and conditions that are required for the given transformation have to be determined.
Concept Introduction:
Acetals:
Acetals are used to protect the
In this reaction acetone is protected as acetal by using ethylene glycol. Acetals are less stable compound.
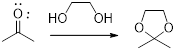
In acidic condition, an aldehyde or a ketone reacts with two molecules of alcohol or a molecule of
General scheme:
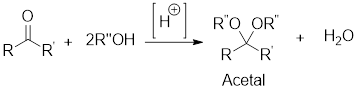
Retro synthetic analysis:
It is the analysis of the synthetic starting materials for a given compound through its precursors or fragments which are known as synthons. The synthons can be obtained by logical cleavage of the
Retro analysis for acetal formation:
The retro synthetic analysis for any given acetal, enable to find that the starting materials for its formation will be alcohol in case of aliphatic acetal or diol in case of cyclic acetal as well as aldehyde or ketone.
Example:
The retro analysis for a cyclic acetal is shown here:
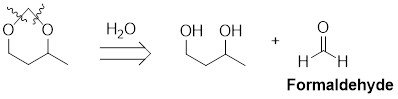
The given retro analysis shows that the starting materials for the formation of the acetal are a diol and formaldehyde.
General scheme:
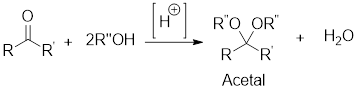
(b)
Interpretation: A plausible mechanism for the given reaction has to be drawn.
Concept Introduction:
Acetals:
Acetals are used to protect the ketone and aldehyde (carbonyl group).
In this reaction acetone is protected as acetal by using ethylene glycol. Acetals are less stable compound.

In acidic condition, an aldehyde or a ketone reacts with two molecules of alcohol or a molecule of diol to form acetal or cyclic acetal respectively.
General scheme:
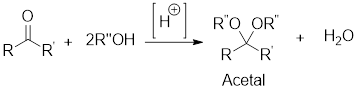
(c)
Interpretation: Based on the given keto-diol precursor, the structure of frontalin has to be drawn.
Concept Introduction:
Acetals:
Acetals are used to protect the ketone and aldehyde (carbonyl group).
In this reaction acetone is protected as acetal by using ethylene glycol. Acetals are less stable compound.

In acidic condition, an aldehyde or a ketone reacts with two molecules of alcohol or a molecule of diol to form acetal or cyclic acetal respectively.
General scheme:
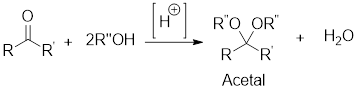
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- PLEASE HELP! URGENT!arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forward
- How many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





