
Chemistry: Atoms First
3rd Edition
ISBN: 9781259923142
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19.3, Problem 1PPC
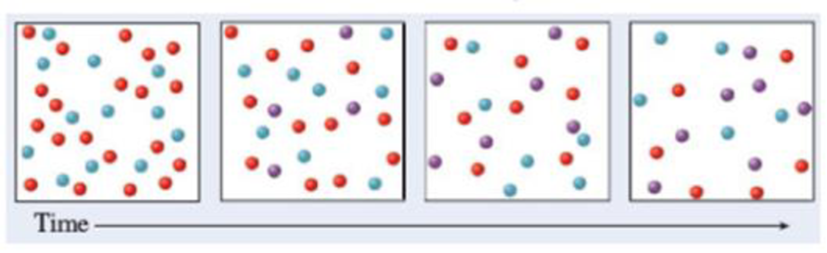
The diagrams represent a system that initially consists of reactants A (red) and B (blue), which react to form product C (purple). Write the balanced chemical equation that corresponds to the reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Experiment:
Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below.
Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization.
Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C
Sample C-Cool the third sample in an ice-bath to 0-2 °C
Results:
weight after recrystalization and melting point temp.
A=0.624g,102-115°
B=0.765g, 80-105°
C=1.135g, 77-108
What is the percent yield of A,B, and C.
Rel. Intensity
Q
1. Which one of the following is true of the compound
whose mass spectrum is shown
here? Explain how you decided.
100
a) It contains chlorine.
b) It contains bromine.
c) It contains neither chlorine nor bromine.
80-
60-
40-
20-
0.0
0.0
TT
40
80
120
160
m/z
2. Using the Table of IR Absorptions how could you
distinguish between these two
compounds in the IR?
What absorbance would one compound have that the
other compound does not?
HO
CI
Illustrate reaction mechanisms of
alkenes with water in the presence of
H2SO4, detailing each step of the
process. Please show steps of
processing. Please do both, I will
thumb up for sure
#1
#3
Chapter 19 Solutions
Chemistry: Atoms First
Ch. 19.3 - Prob. 19.1WECh. 19.3 - Write the rate expressions for each of the...Ch. 19.3 - Write the balanced equation corresponding to the...Ch. 19.3 - The diagrams represent a system that initially...Ch. 19.3 - Consider the reaction 4NO2(g)+O2(g)2N2O5(g) At a...Ch. 19.3 - Consider the reaction 4PH3(g)P4(g)+6H2(g) At a...Ch. 19.3 - Prob. 2PPBCh. 19.3 - Prob. 2PPCCh. 19.3 - Prob. 19.3.1SRCh. 19.3 - Prob. 19.3.2SR
Ch. 19.4 - The gas-phase reaction of nitric oxide with...Ch. 19.5 - Calculate the rate constant for the first-order...Ch. 19.5 - Prob. 19.7WECh. 19.5 - The reaction 2A B is second order in A with a rate...Ch. 19.5 - Prob. 7PPBCh. 19.5 - Prob. 19.5.4SRCh. 19.7 - Prob. 19.11WECh. 19.7 - Prob. 11PPACh. 19.7 - Prob. 11PPBCh. 19.7 - Consider the gas-phase reaction of nitric oxide...Ch. 19.7 - Prob. 12PPBCh. 19 - The rate of a reaction in which the reactant...Ch. 19 - The rate of a reaction in which the reactant...Ch. 19 - The rate of a reaction in which the reactant...Ch. 19 - Increasing the temperature of a reaction increases...Ch. 19 - Define activation energy. What role does...Ch. 19 - Sketch a potential energy versus reaction progress...Ch. 19 - The reaction H + H2 H2 + H has been studied for...Ch. 19 - What is meant by the rate of a chemical reaction?...Ch. 19 - Distinguish between average rate and instantaneous...Ch. 19 - What are the advantages of measuring the initial...Ch. 19 - Prob. 19.7QPCh. 19 - Consider the reaction N2(g)+3H2(g)2NH3(g) Suppose...Ch. 19 - Prob. 19.9QPCh. 19 - Prob. 19.10QPCh. 19 - Prob. 19.11QPCh. 19 - Prob. 19.12QPCh. 19 - Prob. 19.13QPCh. 19 - What are the units for the rate constants of...Ch. 19 - Consider the zeroth-order reaction: A product....Ch. 19 - Prob. 19.16QPCh. 19 - Prob. 19.17QPCh. 19 - Prob. 19.18QPCh. 19 - Prob. 19.19QPCh. 19 - Prob. 19.20QPCh. 19 - Prob. 19.21QPCh. 19 - Prob. 19.22QPCh. 19 - Prob. 19.23QPCh. 19 - Prob. 19.24QPCh. 19 - Prob. 19.25QPCh. 19 - Prob. 19.26QPCh. 19 - Prob. 19.27QPCh. 19 - Prob. 19.28QPCh. 19 - Prob. 19.29QPCh. 19 - Prob. 19.30QPCh. 19 - Prob. 19.31QPCh. 19 - The rate constant for the second-order reaction...Ch. 19 - Prob. 19.33QPCh. 19 - Consider the first-order reaction X Y shown here,...Ch. 19 - Prob. 19.35QPCh. 19 - Consider the first-order reaction A B in which A...Ch. 19 - Prob. 19.37QPCh. 19 - Prob. 19.38QPCh. 19 - Prob. 19.39QPCh. 19 - Prob. 19.40QPCh. 19 - Prob. 19.41QPCh. 19 - Prob. 19.42QPCh. 19 - Prob. 19.43QPCh. 19 - Prob. 19.44QPCh. 19 - Prob. 19.45QPCh. 19 - The rate at which tree crickets chirp is 2.0 102...Ch. 19 - Prob. 19.47QPCh. 19 - The activation energy for the denaturation of a...Ch. 19 - Variation of the rate constant with temperature...Ch. 19 - Prob. 19.50QPCh. 19 - Prob. 19.51QPCh. 19 - Prob. 19.52QPCh. 19 - Prob. 19.53QPCh. 19 - What is an elementary step? What is the...Ch. 19 - Prob. 19.55QPCh. 19 - Determine the molecularity, and write the rate law...Ch. 19 - What is the rate-determining step of a reaction?...Ch. 19 - Prob. 19.58QPCh. 19 - Prob. 19.59QPCh. 19 - Classify each of the following elementary steps as...Ch. 19 - Prob. 19.61QPCh. 19 - Prob. 19.62QPCh. 19 - Prob. 19.63QPCh. 19 - Prob. 19.64QPCh. 19 - Prob. 19.65QPCh. 19 - What are the characteristics of a catalyst?Ch. 19 - Prob. 19.67QPCh. 19 - Prob. 19.68QPCh. 19 - The concentrations of enzymes in cells are usually...Ch. 19 - Prob. 19.70QPCh. 19 - Prob. 19.71QPCh. 19 - Prob. 19.72QPCh. 19 - Prob. 19.73QPCh. 19 - Prob. 19.74QPCh. 19 - Prob. 19.75QPCh. 19 - In a certain industrial process involving a...Ch. 19 - Prob. 19.77QPCh. 19 - Prob. 19.78QPCh. 19 - Explain why most metals used in catalysis arc...Ch. 19 - Prob. 19.80QPCh. 19 - Prob. 19.81QPCh. 19 - Prob. 19.82QPCh. 19 - Prob. 19.83QPCh. 19 - Prob. 19.84QPCh. 19 - The bromination of acetone is acid-catalyzed. The...Ch. 19 - The decomposition of N2O to N2 and O2 is a...Ch. 19 - Prob. 19.87QPCh. 19 - Prob. 19.88QPCh. 19 - The integrated rate law for the zeroth-order...Ch. 19 - Prob. 19.90QPCh. 19 - Prob. 19.91QPCh. 19 - Prob. 19.92QPCh. 19 - The reaction of G2 with E2 to form 2EG is...Ch. 19 - Prob. 19.94QPCh. 19 - Prob. 19.95QPCh. 19 - Prob. 19.96QPCh. 19 - Strictly speaking, the rate law derived for the...Ch. 19 - Prob. 19.98QPCh. 19 - The decomposition of dinitrogen pentoxide has been...Ch. 19 - Prob. 19.100QPCh. 19 - Prob. 19.101QPCh. 19 - Prob. 19.102QPCh. 19 - To prevent brain damage, a standard procedure is...Ch. 19 - Prob. 19.104QPCh. 19 - Prob. 19.105QPCh. 19 - Prob. 19.106QPCh. 19 - Prob. 19.107QPCh. 19 - Prob. 19.108QPCh. 19 - Prob. 19.109QPCh. 19 - Prob. 19.110QPCh. 19 - (a) What can you deduce about the activation...Ch. 19 - Prob. 19.112QPCh. 19 - Prob. 19.113QPCh. 19 - Prob. 19.114QPCh. 19 - Prob. 19.115QPCh. 19 - Prob. 19.116QPCh. 19 - Prob. 19.117QPCh. 19 - Prob. 19.118QPCh. 19 - Prob. 19.119QPCh. 19 - Prob. 19.120QPCh. 19 - Prob. 19.121QPCh. 19 - Prob. 19.122QPCh. 19 - Consider the following potential energy profile...Ch. 19 - Prob. 19.124QPCh. 19 - Prob. 19.125QPCh. 19 - Prob. 19.126QPCh. 19 - Prob. 19.127QPCh. 19 - Prob. 19.128QPCh. 19 - The following expression shows the dependence of...Ch. 19 - Prob. 19.130QPCh. 19 - The rale constant for the gaseous reaction H2(g) +...Ch. 19 - Prob. 19.132QPCh. 19 - Prob. 19.133QPCh. 19 - At a certain elevated temperature, ammonia...Ch. 19 - Prob. 19.135QPCh. 19 - The rate of a reaction was followed by the...Ch. 19 - Prob. 19.137QPCh. 19 - Prob. 19.138QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forwardCS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forward
- CS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forward
- Collagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forwardיווי 10 20 30 40 50 60 70 3.5 3 2.5 2 1.5 1 [ppm] 3.5 3 2.5 2 1.5 1 6 [ppm] 1 1.5 -2.5 3.5arrow_forward2H2S(g)+3O2(g)→2SO2(g)+2H2O(g) A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion. Question Which of the following predicts the theoretical yield of SO2(g) from the reaction? Responses 1.2 g Answer A: 1.2 grams A 41 g Answer B: 41 grams B 77 g Answer C: 77 grams C 154 g Answer D: 154 grams Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY