
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (Chs 1-42) Plus Mastering Physics with Pearson eText -- Access Card Package (4th Edition)
4th Edition
ISBN: 9780133953145
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 8EAP
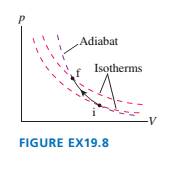
Draw a first-law bar chart (see Figure 19.12) for the gas process
in FIGURE EX19.8.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
Review the data in Data Table 1 and examine the standard deviations and 95% Margin of Error calculations from Analysis Questions 3 and 4 for the Acceleration of the 1st Based on this information, explain whether Newton’s Second Law of Motion, Equation 1, was verified for your 1st Angle.
Equation: SF=ma
Please help with explaining the information I collected from a lab and how it relates to the equation and Newton's Second Law. This will help with additional tables in the lab. Thanks!
Please solve and answer the problem step by step with explanations along side each step stating what's been done correctly please. Thank you!! ( preferably type out everything)
Chapter 19 Solutions
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (Chs 1-42) Plus Mastering Physics with Pearson eText -- Access Card Package (4th Edition)
Ch. 19 - Prob. 1CQCh. 19 - Do (a) temperature, (b) heat, and (c) thermal...Ch. 19 - Prob. 3CQCh. 19 - You need to raise the temperature of a gas by...Ch. 19 - Prob. 5CQCh. 19 - Prob. 6CQCh. 19 - FIGURE Q19.7 shows two different processes taking...Ch. 19 - FIGURE Q19.8 shows two different processes taking...Ch. 19 - The gas cylinder in FIGURE Q19.9 is a rigid...Ch. 19 - The gas cylinder in FIGURE Q19.10 is well...
Ch. 19 - The gas cylinder in FIGURE Q19.11 is well...Ch. 19 - How much work is done on the gas in the process...Ch. 19 - Prob. 2EAPCh. 19 - Prob. 3EAPCh. 19 - A 2000 cm3 container holds 0.10 mol of helium gas...Ch. 19 - Prob. 5EAPCh. 19 - Prob. 6EAPCh. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - 9. Draw a first-law bar chart (see Figure 19.12)...Ch. 19 - Prob. 10EAPCh. 19 - J of work are done on a system in a process that...Ch. 19 - How much heat energy must be added to a...Ch. 19 - Prob. 13EAPCh. 19 - Prob. 14EAPCh. 19 - Prob. 15EAPCh. 19 - Prob. 16EAPCh. 19 - One way you keep from overheating is by...Ch. 19 - Prob. 18EAPCh. 19 - Two cars collide head-on while each is traveling...Ch. 19 - An experiment measures the temperature of a 500 g...Ch. 19 - 30 g of copper pellets are removed from a 300°C...Ch. 19 - A 750 g aluminum pan is removed from the stove and...Ch. 19 - A 50.0 g thermometer is used to measure the...Ch. 19 - A 500 g metal sphere is heated to 300°C, then...Ch. 19 - A 65 cm3 block of iron is removed from an 800°C...Ch. 19 - Prob. 26EAPCh. 19 - A container holds 1.0 g of oxygen at a pressure of...Ch. 19 - The volume of a gas is halved during an adiabatic...Ch. 19 - Prob. 29EAPCh. 19 - Prob. 30EAPCh. 19 - Prob. 31EAPCh. 19 - Prob. 32EAPCh. 19 - Prob. 33EAPCh. 19 - Prob. 34EAPCh. 19 - Prob. 35EAPCh. 19 - What maximum power can be radiated by a...Ch. 19 - Radiation from the head is a major source of heat...Ch. 19 - Prob. 38EAPCh. 19 - Prob. 39EAPCh. 19 - Prob. 40EAPCh. 19 - Prob. 41EAPCh. 19 - Prob. 42EAPCh. 19 - Prob. 43EAPCh. 19 - The specific heat of most solids is nearly...Ch. 19 - Prob. 45EAPCh. 19 - Prob. 46EAPCh. 19 - Prob. 47EAPCh. 19 - Prob. 48EAPCh. 19 - .0 mol of gas are at 30°C and a pressure of 1.5...Ch. 19 - A 6.0-cm-diameter cylinder of nitrogen gas has a...Ch. 19 - Prob. 51EAPCh. 19 - An ideal-gas process is described by p = cV 1/2 ,...Ch. 19 - Prob. 53EAPCh. 19 - Prob. 54EAPCh. 19 - Prob. 55EAPCh. 19 - Prob. 56EAPCh. 19 - Prob. 57EAPCh. 19 - .10 mol of nitrogen gas follow the two processes...Ch. 19 - Prob. 59EAPCh. 19 - Prob. 60EAPCh. 19 - Prob. 61EAPCh. 19 - Prob. 62EAPCh. 19 - Prob. 63EAPCh. 19 - Prob. 64EAPCh. 19 - Prob. 65EAPCh. 19 - Prob. 66EAPCh. 19 - Prob. 67EAPCh. 19 - Prob. 68EAPCh. 19 - Prob. 69EAPCh. 19 - A cylindrical copper rod and an iron rod with...Ch. 19 - Prob. 71EAPCh. 19 - Prob. 72EAPCh. 19 - Prob. 73EAPCh. 19 - Prob. 74EAPCh. 19 - Prob. 75EAPCh. 19 - Prob. 76EAPCh. 19 - Prob. 77EAPCh. 19 - Prob. 78EAPCh. 19 - Prob. 79EAPCh. 19 - Prob. 80EAPCh. 19 - Prob. 81EAPCh. 19 - Prob. 82EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Kirchoff's Laws. A circuit contains 3 known resistors, 2 known batteries, and 3 unknown currents as shown. Assume the current flows through the circuit as shown (this is our initial guess, the actual currents may be reverse). Use the sign convention that a potential drop is negative and a potential gain is positive. E₂ = 8V R₁₁ = 50 R₂ = 80 b с w 11 www 12 13 E₁ = 6V R3 = 20 a) Apply Kirchoff's Loop Rule around loop abefa in the clockwise direction starting at point a. (2 pt). b) Apply Kirchoff's Loop Rule around loop bcdeb in the clockwise direction starting at point b. (2 pt). c) Apply Kirchoff's Junction Rule at junction b (1 pt). d) Solve the above 3 equations for the unknown currents I1, 12, and 13 and specify the direction of the current around each loop. (5 pts) I1 = A 12 = A 13 = A Direction of current around loop abef Direction of current around loop bcde (CW or CCW) (CW or CCW)arrow_forwardNo chatgpt pls will upvotearrow_forward4.) The diagram shows the electric field lines of a positively charged conducting sphere of radius R and charge Q. A B Points A and B are located on the same field line. A proton is placed at A and released from rest. The magnitude of the work done by the electric field in moving the proton from A to B is 1.7×10-16 J. Point A is at a distance of 5.0×10-2m from the centre of the sphere. Point B is at a distance of 1.0×10-1 m from the centre of the sphere. (a) Explain why the electric potential decreases from A to B. [2] (b) Draw, on the axes, the variation of electric potential V with distance r from the centre of the sphere. R [2] (c(i)) Calculate the electric potential difference between points A and B. [1] (c(ii)) Determine the charge Q of the sphere. [2] (d) The concept of potential is also used in the context of gravitational fields. Suggest why scientists developed a common terminology to describe different types of fields. [1]arrow_forward
- 3.) The graph shows how current I varies with potential difference V across a component X. 904 80- 70- 60- 50- I/MA 40- 30- 20- 10- 0+ 0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 VIV Component X and a cell of negligible internal resistance are placed in a circuit. A variable resistor R is connected in series with component X. The ammeter reads 20mA. 4.0V 4.0V Component X and the cell are now placed in a potential divider circuit. (a) Outline why component X is considered non-ohmic. [1] (b(i)) Determine the resistance of the variable resistor. [3] (b(ii)) Calculate the power dissipated in the circuit. [1] (c(i)) State the range of current that the ammeter can measure as the slider S of the potential divider is moved from Q to P. [1] (c(ii)) Describe, by reference to your answer for (c)(i), the advantage of the potential divider arrangement over the arrangement in (b).arrow_forward1.) Two long parallel current-carrying wires P and Q are separated by 0.10 m. The current in wire P is 5.0 A. The magnetic force on a length of 0.50 m of wire P due to the current in wire Q is 2.0 × 10-s N. (a) State and explain the magnitude of the force on a length of 0.50 m of wire Q due to the current in P. [2] (b) Calculate the current in wire Q. [2] (c) Another current-carrying wire R is placed parallel to wires P and Q and halfway between them as shown. wire P wire R wire Q 0.05 m 0.05 m The net magnetic force on wire Q is now zero. (c.i) State the direction of the current in R, relative to the current in P.[1] (c.ii) Deduce the current in R. [2]arrow_forward2.) A 50.0 resistor is connected to a cell of emf 3.00 V. The voltmeter and the ammeter in the circuit are ideal. V A 50.00 (a) The current in the ammeter is 59.0 mA. Calculate the internal resistance of the cell. The circuit is changed by connecting another resistor R in parallel to the 50.0 resistor. V A 50.00 R (b) Explain the effect of this change on R is made of a resistive wire of uniform cross-sectional area 3.1 × 10-8 m², resistivity 4.9 × 10-70m and length L. The resistance of R is given by the equation R = KL where k is a constant. (b.i) the reading of the ammeter. [2] (b.ii) the reading of the voltmeter. [2] (c) Calculate k. State an appropriate unit for your answer. [3] [2]arrow_forward
- No chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forwardA rod 12.0 cm long is uniformly charged and has a total charge of -20.0 μc. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center. 361000 ☑ magnitude What is the general expression for the electric field along the axis of a uniform rod? N/C direction toward the rodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY