
Principles of Chemistry: A Molecular Approach Plus Mastering Chemistry with eText -- Access Card Package (3rd Edition) (New Chemistry Titles from Niva Tro)
3rd Edition
ISBN: 9780321971166
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19, Problem 62E
Interpretation Introduction
Interpretation:
The difference in energy released by the two given decay processes of 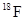 is to be calculated.
is to be calculated.
Concept introduction: When a stable nuclide is formed from its component particles, mass is converted to energy. The difference in mass is known as mass defect and energy corresponding to the mass defect is known as binding energy.
To determine: The difference in energy released by the two given decay processes of 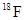
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Which one of the following is trans-1-tert-butyl-3-methylcyclohexane in its most stable
conformation? (NOTE: Correct answer must be trans- and must have a 1,3-arrangement of groups.)
C(CH3)3
CH₁₂
A
H,C
D
H₂C
C(CH)
C(CH3)3
C
B
CH
C(CH)
C(CH3)3
E
Predict the Product. Predict the major organic product for the following reaction:
None
Chapter 19 Solutions
Principles of Chemistry: A Molecular Approach Plus Mastering Chemistry with eText -- Access Card Package (3rd Edition) (New Chemistry Titles from Niva Tro)
Ch. 19 - Prob. 19.1PCh. 19 - Prob. 19.2PCh. 19 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Prob. 19.5PCh. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 1SAQCh. 19 - Prob. 2SAQCh. 19 - Prob. 3SAQ
Ch. 19 - Prob. 4SAQCh. 19 - Prob. 5SAQCh. 19 - Prob. 6SAQCh. 19 - Prob. 7SAQCh. 19 - Prob. 8SAQCh. 19 - Prob. 9SAQCh. 19 - Prob. 10SAQCh. 19 - Prob. 1ECh. 19 - Prob. 2ECh. 19 - Prob. 3ECh. 19 - Prob. 4ECh. 19 - Prob. 5ECh. 19 - Prob. 6ECh. 19 - Prob. 7ECh. 19 - Prob. 8ECh. 19 - Prob. 9ECh. 19 - Prob. 10ECh. 19 - Prob. 11ECh. 19 - Prob. 12ECh. 19 - Prob. 13ECh. 19 - Prob. 14ECh. 19 - Prob. 15ECh. 19 - Prob. 16ECh. 19 - Prob. 17ECh. 19 - Prob. 18ECh. 19 - Prob. 19ECh. 19 - Prob. 20ECh. 19 - Prob. 21ECh. 19 - Prob. 22ECh. 19 - Prob. 23ECh. 19 - Prob. 24ECh. 19 - Prob. 25ECh. 19 - Prob. 26ECh. 19 - Prob. 27ECh. 19 - Prob. 28ECh. 19 - Prob. 29ECh. 19 - Prob. 30ECh. 19 - Prob. 31ECh. 19 - Prob. 32ECh. 19 - Prob. 33ECh. 19 - Prob. 34ECh. 19 - Prob. 35ECh. 19 - Prob. 36ECh. 19 - Prob. 37ECh. 19 - Prob. 38ECh. 19 - Prob. 39ECh. 19 - Prob. 40ECh. 19 - Prob. 41ECh. 19 - Prob. 42ECh. 19 - Prob. 43ECh. 19 - Prob. 44ECh. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Prob. 47ECh. 19 - Prob. 48ECh. 19 - Prob. 49ECh. 19 - Prob. 50ECh. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - Prob. 53ECh. 19 - Prob. 54ECh. 19 - Prob. 55ECh. 19 - Prob. 56ECh. 19 - Prob. 57ECh. 19 - Prob. 58ECh. 19 - Prob. 59ECh. 19 - Prob. 60ECh. 19 - Prob. 61ECh. 19 - Prob. 62ECh. 19 - Prob. 63ECh. 19 - Prob. 64ECh. 19 - Prob. 65ECh. 19 - Prob. 66ECh. 19 - Prob. 67ECh. 19 - Prob. 68ECh. 19 - Prob. 69E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Which one of the following is the lowest energy, most stable conformation of 1-bromopropane? H H H H H H H H CH3 HH Br H CH3 b b b b b CH3 A Br Br H H B CH3 Br H C H H H D CH3 H Br H E Harrow_forwardIn evolution, migration refers to the movement of alleles between populations. In your drawings, compare and contrast migration in evolutionary terms vs. in ecological terms. True Falsearrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 31 I 1 :0: O: C 1 1 H Na Select to Add Arrows CH3CH2CCNa 1arrow_forward
- Blackboard app.aktiv.com X Organic Chemistry II Lecture (mx Aktiv Learning App Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 25 of 35 Select to Edit Arrows CH3CH2OK, CH3CH2OH L Gemini M 31 0:0 :0: 5x Undo Reset Done :0: Harrow_forwardI have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Problem 17 of 35 1. CH3CH2Li O H 2. Neutralizing work-up @ Atoms, Bonds and Rings Draw or tap a new boarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY