
Conceptual Physical Science Plus Mastering Physics with Pearson eText -- Access Card Package (6th Edition)
6th Edition
ISBN: 9780134060484
Author: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 32TAR
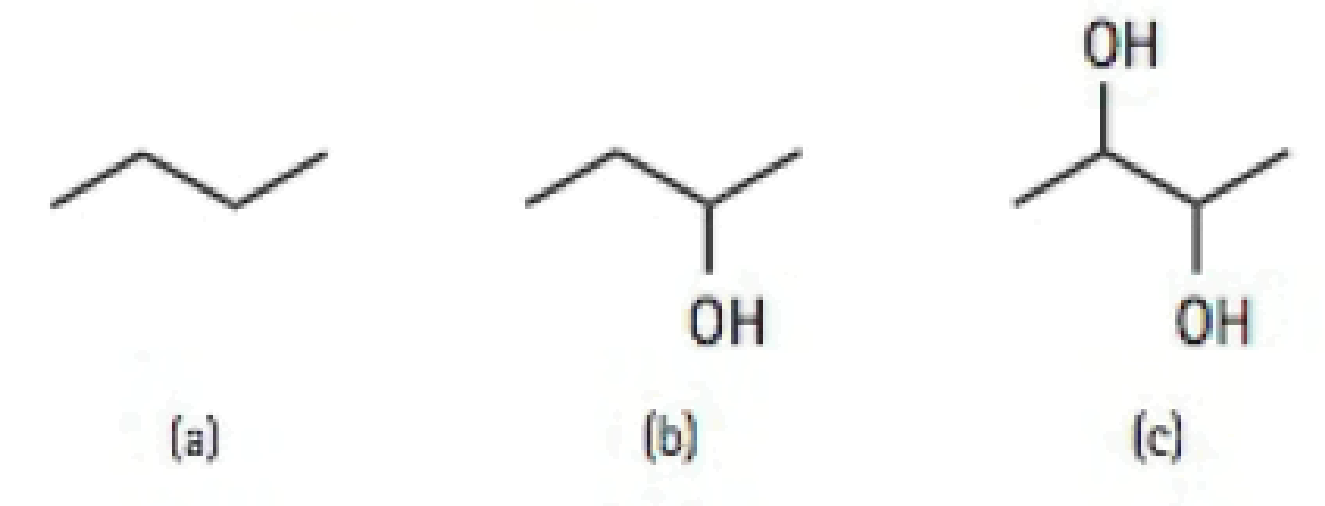
Rank the following organic molecules in order of increasing solubility in water:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
SARET CRKS AUTOWAY
12. A stone is dropped from the top of a cliff. It is seen to hit the ground below
after 3.55 s. How high is the cliff?
13. A ball is dropped from rest at the top of a building that is 320 m tall. Assuming
no air resistance, what is the speed of the ball just before it strikes the ground?
14. Estimate (a) how long it took King Kong to fall straight down from the top
of the Empire State Building (280m high), and (b) his velocity just before
"landing".
Useful equations
For Constant Velocity:
V =>
D
X = V₁t + Xo
For Constant Acceleration:
Vr = V + at
X = Xo+Vot +
v=V+2a(X-Xo)
\prom = V +V
V velocity
t = time
D Distance
X = Final Position
Xo Initial Position
V = Final Velocity
Vo Initial Velocity
a = acceleration
For free fall
Yf
= Final Position
Yo Initial Position
g = 9.80
m
$2
For free fall:
V = V + gt
Y=Yo+Vo t +
+gt
V,² = V₁²+2g (Y-Yo)
V+Vo
Vprom=
2
6
Solve the problems
A 11 kg weight is attached to a spring with constant k = 99 N/m and subjected to an external force
F(t) =-704 sin(5t). The weight is initially displaced 4 meters above equilibrium and given an
upward velocity of 5 m/s. Find its displacement for t> 0.
y(t)
ון
Chapter 19 Solutions
Conceptual Physical Science Plus Mastering Physics with Pearson eText -- Access Card Package (6th Edition)
Ch. 19 - How do two structural isomers differ from each...Ch. 19 - How are two structural isomers similar to each...Ch. 19 - What physical property of hydrocarbons is used in...Ch. 19 - What types of hydrocarbons are more abundant in...Ch. 19 - To how many atoms is a saturated carbon atom...Ch. 19 - What is the difference between a saturated...Ch. 19 - How many multiple bonds must a hydrocarbon have in...Ch. 19 - What kind of ring do aromatic compounds contain?Ch. 19 - What is a heteroatom?Ch. 19 - Why do heteroatoms make such a difference in the...
Ch. 19 - Why are low-formula-mass alcohols soluble in...Ch. 19 - What distinguishes an alcohol from a phenol?Ch. 19 - What distinguishes an alcohol from an ether?Ch. 19 - Which heteroatom is characteristic of an amine?Ch. 19 - Do amines tend to be acidic, neutral, or basic?Ch. 19 - Are alkaloids found in nature?Ch. 19 - What are some examples of alkaloids?Ch. 19 - Which elements make up the carbonyl group?Ch. 19 - How are ketones and aldehydes related to each...Ch. 19 - How are amides and carboxylic acids related to...Ch. 19 - From what naturally occurring compound is aspirin...Ch. 19 - What happens to the double bond of a monomer...Ch. 19 - What is released in the formation of a...Ch. 19 - Why is plastic wrap made of polyvinylidene...Ch. 19 - Prob. 25RCQCh. 19 - Rank the following molecules in order of the phase...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - What property of carbon allows for the formation...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - Draw all the structural isomers for hydrocarbons...Ch. 19 - How many structural isomers are shown here?Ch. 19 - According to Figure 19.3, which has the higher...Ch. 19 - The temperatures in a fractionating tower at an...Ch. 19 - Prob. 40ECh. 19 - Do heavier hydrocarbons tend to produce more or...Ch. 19 - What do these two structures have in common?Ch. 19 - What do the compounds cyclopropane and propene...Ch. 19 - What are the chemical formulas for the following...Ch. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Identify the following functional groups in this...Ch. 19 - What must be added to a double bond to transform...Ch. 19 - What do phenols and carboxylic acids have in...Ch. 19 - What is the difference between a ketone and an...Ch. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - What is the percent volume of water in 80-proof...Ch. 19 - One of the skin-irritating components of poison...Ch. 19 - Cetyl alcohol, C16H34O, is a common ingredient of...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - The phosphoric acid salt of caffeine has the...Ch. 19 - Prob. 59ECh. 19 - In water, does the following molecule act as an...Ch. 19 - If you saw the label phenylephrine-HCl on a...Ch. 19 - The amino acid lysine is shown below. What...Ch. 19 - Prob. 63ECh. 19 - Suggest an explanation why aspirin has a sour...Ch. 19 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 19 - What products are formed upon the reaction of...Ch. 19 - The disodium salt of ethylenediaminetetraacetic...Ch. 19 - Would you expect polypropylene to be more dense or...Ch. 19 - Hydrocarbons release a lot of energy when ignited....Ch. 19 - The polymer styrene-butadiene rubber (SBR), shown...Ch. 19 - Citral and camphor are both 10-carbon odoriferous...Ch. 19 - Many of the natural product molecules synthesized...Ch. 19 - The solvent diethyl ether can be mixed with water...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - How many structural isomers are there for...Ch. 19 - Which contains more hydrogen atoms: a five-carbon...Ch. 19 - Prob. 4RATCh. 19 - Why might a high-formula-mass alcohol be insoluble...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Explain why caprylic acid, CH3(CH2)6 COOH,...Ch. 19 - How many oxygen atoms are bonded to the carbon of...Ch. 19 - One solution to the problem of our overflowing...Ch. 19 - Which would you expect to be more viscous: a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
The distances you obtained in Question 3 are for only one side of the ridge. Assuming that a ridge spreads equa...
Applications and Investigations in Earth Science (9th Edition)
The glycine cleavage system is a group of four enzymes that together catalyze the following reaction: glycine+T...
Organic Chemistry (8th Edition)
In cats, tortoiseshell coat color appears in females. A tortoiseshell coat has patches of dark brown fur and pa...
Genetic Analysis: An Integrated Approach (3rd Edition)
47. Balance each chemical equation.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 7. A race car accelerates from rest to 55 m s-1 in 5.0 seconds. The acceleration of the car Is m s-² 8. An object's speed increases uniformly from 10.5 km per hour to 99.8 km per hour in 2.41 seconds. Calculate the acceleration in m s-2 and express your answer to three significant figures. 9. The acceleration-time graph of a car is shown below. The initial speed of the car is 5.0 m s-1. # Acceleration (ms) 12 8.0- 4.0- 2.0 4.0 6.0 Time (s) Calculate the velocity of the car at t = 4.0 s. 3arrow_forwardNo chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forward
- Problem Seven. A football receiver running straight downfield at 5.60 m/s is 11.5 m in front of the quarterback when a pass is thrown downfield at an angle of 35.0° horizon. above the 8.) If the receiver never changes speed and the ball is caught at the same height from which it was thrown, find the distance between the quarterback and the receiver when the catch is made. (A) 21.3 (B) 17.8 (C) 18.8 (D) 19.9 (E) 67.5arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forward
- Please view both photos, and answer the question correctly please. Thank you!!arrow_forwardA thrown brick hits a window, but doesn't break it. Instead it reverses direction and ends down on the ground below the window. Since the brick didn't break the glass, we know: О The force of the brick on the glass > the force of the glass on the brick. О The force of the brick on the glass the force of the glass on the brick. = О The force of the brick on the glass < the force of the glass on the brick. О The brick didn't slow down as it broke the glass.arrow_forwardAlexandra (wearing rubber boots for traction) is attempting to drag her 32.6-kg Golden Retriever across the smooth ice by applying a horizontal force. What force must she apply to move the dog with a constant speed of 0.950 m/s? ☐ 31.0 lb. ☐ 319 kg. ○ Zero. 32.6 kg.arrow_forward
- The figure shows a graph of the acceleration of an object as a function of the net force acting on it. The mass of this object, in grams, is closest to 11 a(m/s²) 8.0+ 6.0- 4.0- 2.0- 0+ F(N) 0.00 0.50 1.00 ☐ 130 ○ 8000 ☐ 89arrow_forwardValues that are within standard deviations represent measurements that are considered to be near the true value. Review the data from the lab and determine whether your data is within standard deviations. Report, using numerical values, whether your data for each angle is within standard deviations. An acceptable margin of error typically falls between 4% and 8% at the 95% confidence level. Review your data for each angle to determine whether the margin of error is within an acceptable range. Report with numerical values, whether your data for each angle is within an acceptable margin of error. Can you help explain what my data means in terms of the standard deviation and the ME? Thanks!arrow_forwardA sinusoidal wave is propagating along a stretched string that lies along the x-axis. The displacement of the string as a function of time is graphed in (Figure 1) for particles at x = 0 and at x = 0.0900 m. You are told that the two points x = 0 and x = 0.0900 m are within one wavelength of each other. If the wave is moving in the +x-direction, determine the wavelength. If instead the wave is moving in the -x-direction, determine the wavelength. Please show all stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY