
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
8th Edition
ISBN: 9780134595450
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 27P
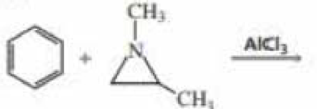
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.
- a. What are the major and minor products of the following reaction?
- b. Would you expect
epoxides to undergo similar reactions?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the equation...see photo
Please see photo
=Naming benzene derivatives
Name these organic compounds:
structure
C1
CH3
name
☐
CH3
ப
C1
×
☐
Chapter 19 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forwardCan you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forward
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY