
Concept explainers
PRACTICE PROBLEM 19.1
(a) Write a mechanism. for all steps of the Claisen condensation that take place when ethyl propanoate reacts with ethoxide ion.
(b) What products form when the reaction mixture is acidified?
Interpretation: The mechanism for all the steps of Claisen condensation when ethyl propanate reacts with ethoxide ion is to be written and when the reaction mixture acidified the product of the reaction is to be determined.
Concept introduction:
The Claisen condensation is the carbon-carbon bond forming reaction and important for the preparing
In Claisen condensation ester of one molecule adds to the carbonyl carbon of the another molecule and formation of
Answer to Problem 1PP
Solution:
(a)
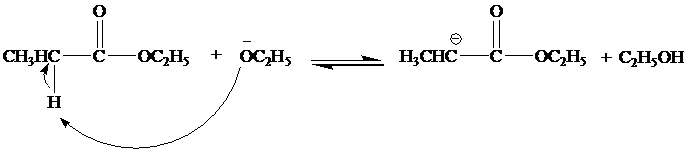
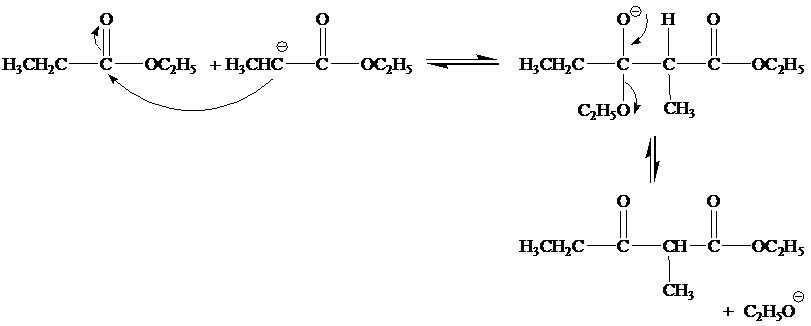

(b)
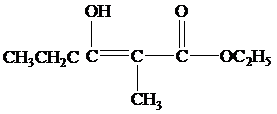
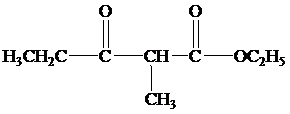
Explanation of Solution
The mechanism for the reaction between ethylproponate and ethoxide ion is as follows:
Step 1
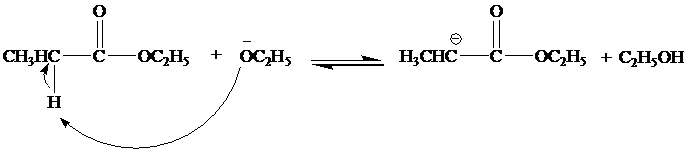
Step 2
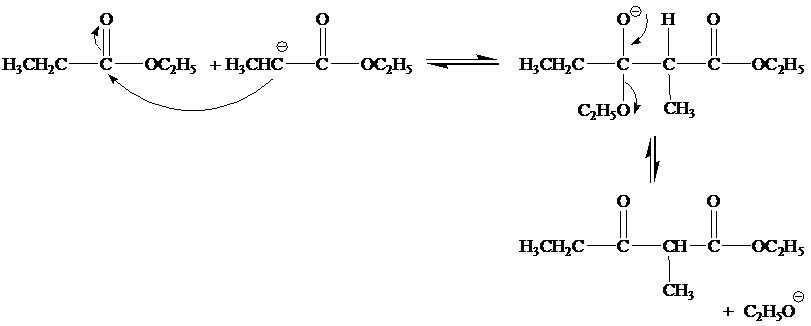
Step 3

(b)
When the reaction mixture is acidified the reaction takes place is as follows;
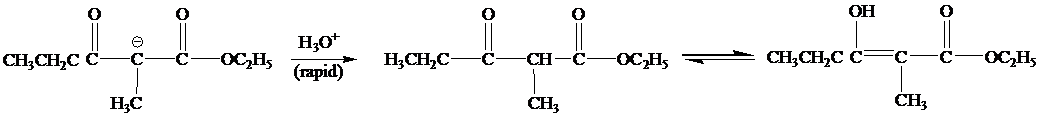
Hence, the product formed when the reaction mixture acidified is as follows;
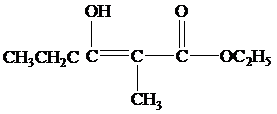
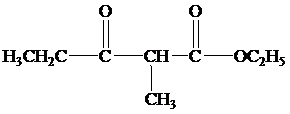

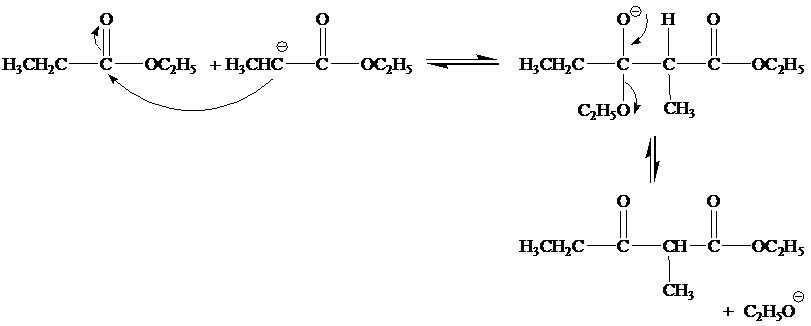

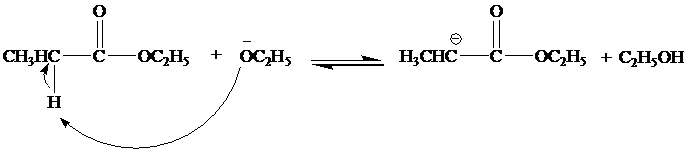
Want to see more full solutions like this?
Chapter 19 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Additional Science Textbook Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Organic Chemistry (8th Edition)
Fundamentals of Physics Extended
Microbiology: An Introduction
Human Biology: Concepts and Current Issues (8th Edition)
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

