
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
11th Edition
ISBN: 9781305081055
Author: Bettelheim, Frederick A.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.8P
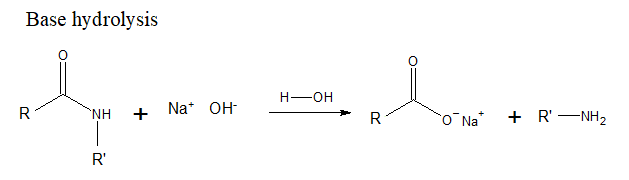
(a)
Interpretation Introduction
Interpretation: The products formed when benzamide, C6H5CONH2is treated with the following set of reagents should be determined.
H2O, NaOH, heat
Concept Introduction: Basic hydrolysis with NaOH causes an acid amide to form sodium salt of the
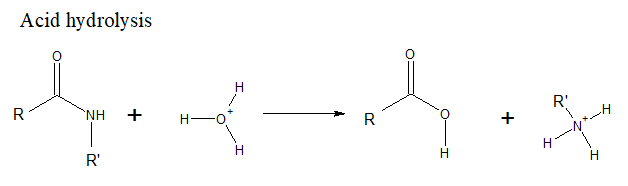
(b)
Interpretation Introduction
Interpretation: The products formed when benzamide, C6H5CONH2is treated with the following set of reagents should be determined.
H2O, HCl, heat
Concept Introduction: Acid hydrolysis with HCl causes an acid amide to form carboxylic acid and amine.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Indicate the names of these compounds (if they exist).
0:
HỌC—NH
CH3CH2-CH2
N
Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic.
NH
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
G
The conjugate base of alkanes is called alkides. Correct?.
Chapter 19 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Similar questions
- Name these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forward
- Name these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning