
(a)
Interpretation:
Synthesis of the given target molecule from phenylmethanol (benzyl alcohol) is to be determined.
Concept introduction:
Acid is converted to ester group by esterification reaction with the help of a strong base like
Answer to Problem 19.75P
Synthesis of the target molecule from phenylmethanol (benzyl alcohol) is
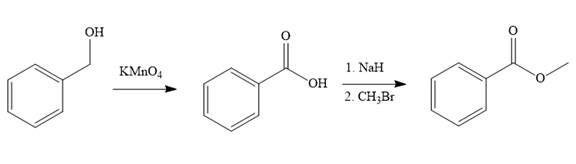
Explanation of Solution
Structure of the target molecule is
In the first step of the reaction, phenylmethanol (benzyl alcohol) is treated with oxidizing agent
In the second step of the reaction, benzoic acid undergoes esterification reaction. In this step, benzoic acid is first treated with a strong base like
The complete synthesis of the target molecule is as shown below.
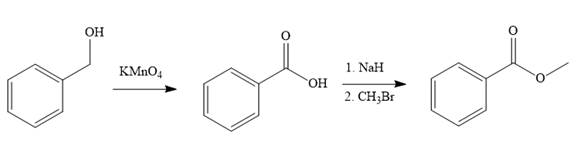
By using the
(b)
Interpretation:
Synthesis of the given target molecule from phenylmethanol (benzyl alcohol) is to be determined.
Concept introduction:
For the conversion of alcohol group to Grignard reagent, alcohol (bad leaving group) is treated with
When Grignard reagent is reacted with
For the removal of water, tertiary alcohol is treated with conc.
The bromination reaction on alkene takes place in presence of
Answer to Problem 19.75P
Synthesis of the target molecule from phenylmethanol (benzyl alcohol) is
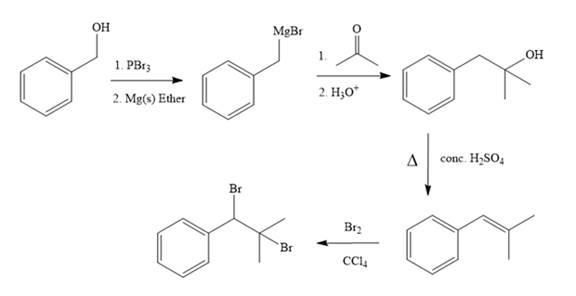
Explanation of Solution
Structure of the target molecule is
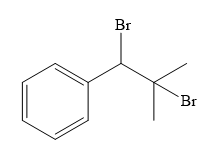
In the first step, the formation of Grignard reagent takes place. In this step, benzyl alcohol is treated with
The complete synthesis of the target molecule is as shown below.
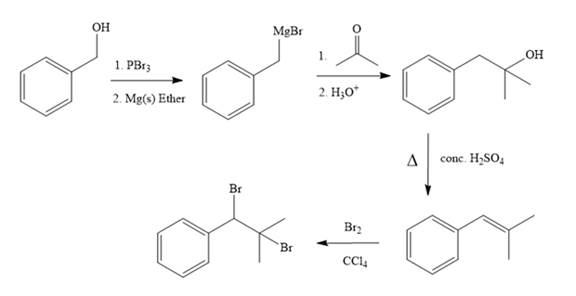
By using the functional group conversion reactions, the synthesis of the target molecule is determined.
(c)
Interpretation:
Synthesis of the given target molecule from phenylmethanol (benzyl alcohol) is to be determined.
Concept introduction:
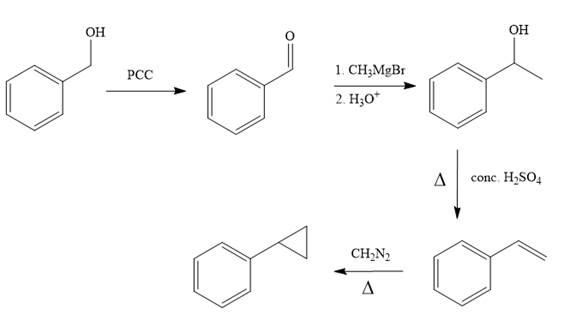
Grignard reagent, in presence of acidic condition, is used for the conversion of aldehyde or ketone to alcohol.
For the removal of water, tertiary alcohol is treated with conc.
Reaction of alkene with diazomethane in presence of heat results in the formation of cyclopropane ring.
Answer to Problem 19.75P
Synthesis of the target molecule from phenylmethanol (benzyl alcohol) is
Explanation of Solution
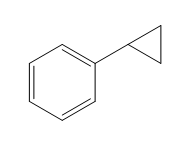
Structure of the target molecule is
In the first step, benzyl alcohol is treated with the oxidizing agent
In the second step, benzaldehyde is treated with Grignard reagent
In the third step, the presence of conc.
In the last step, alkene is treated with diazomethane in presence of heat that results in the formation of the cyclopropane ring.
The complete synthesis of the target molecule is as shown below.
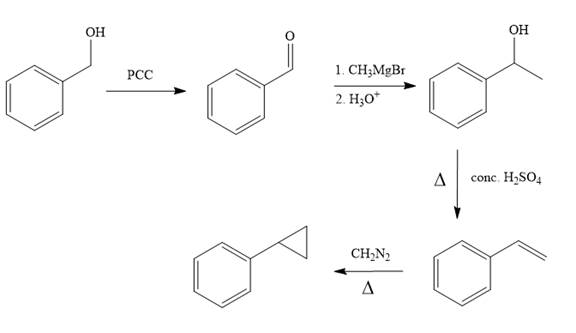
By using the functional group conversion reactions, the synthesis of the target molecule is determined.
(d)
Interpretation:
Synthesis of the given target molecule from phenylmethanol (benzyl alcohol) is to be determined.
Concept introduction:
Grignard reagent, in presence of acidic condition, is used for the conversion of aldehyde or ketone to alcohol.
Answer to Problem 19.75P
Synthesis of the target molecule from phenylmethanol (benzyl alcohol) is
Explanation of Solution
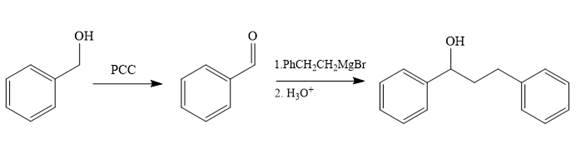
Structure of the target molecule is
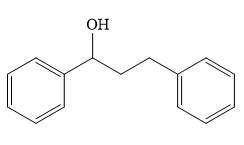
In the first step, benzyl alcohol is treated with the oxidizing agent
In the second step, benzaldehyde is treated with Grignard reagent
The complete synthesis of the target molecule is as shown below.

By using the functional group, conversion reactions synthesis of the target molecule is determined.
Want to see more full solutions like this?
Chapter 19 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- The U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forwardSuppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forward
- A pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forwardNonearrow_forward
- Draw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





