
(a)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
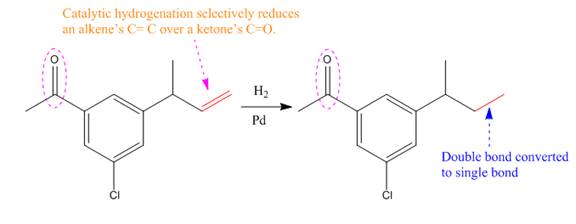
Explanation of Solution
The given reaction is
The given reaction condition correlates to catalytic hydrogenation; it reduces alkene’s C=C over ketone’s C=O. Thus, the
Therefore, the product of the given reaction is
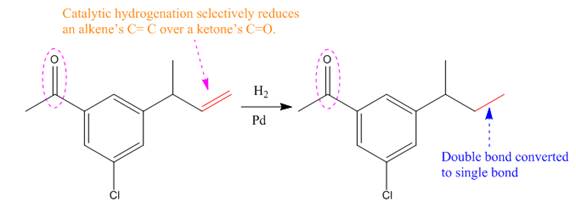
The product of the given reaction is predicted using the given reaction conditions.
(b)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
Answer to Problem 19.54P
The product of the given reaction is
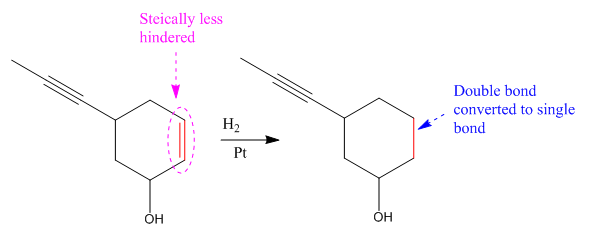
Explanation of Solution
The given reaction
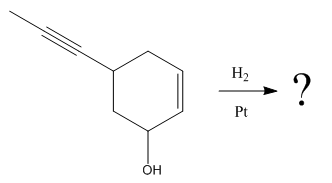
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction have two sites for catalytic hydrogenation, one C=C bond and another
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
In the given reaction, the C=C bond is sterically less hindered; therefore the

The product of the given reaction is predicted using given reaction conditions.
(c)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
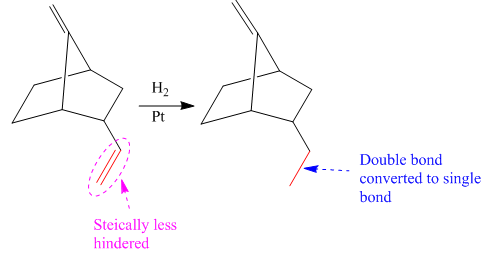
Explanation of Solution
The given reaction is
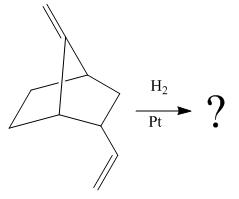
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction have two sites for catalytic hydrogenation, two C=C bonds.
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
In the given reaction, the C=C bond is at bottom is sterically less hindered; therefore the

The product of the given reaction is predicted using the given reaction conditions.
(d)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
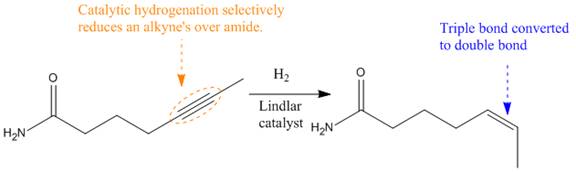
Explanation of Solution
The given reaction is
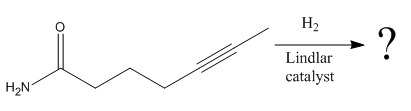
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one
The functional groups in alkenes, alkynes, and aldehydes can be selectively reduced over those in ketones, nitriles, and amides. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
(e)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
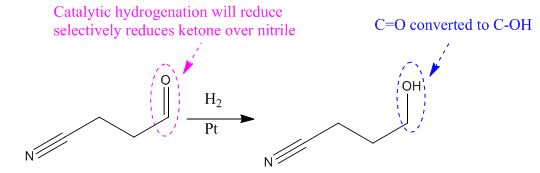
Explanation of Solution
The given reaction is
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one C=C bond and another the nitrile group.
The functional group ketones can be selectively reduced over nitriles. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
(f)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
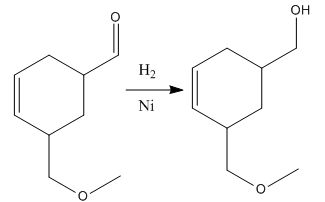
Explanation of Solution
The given reaction is
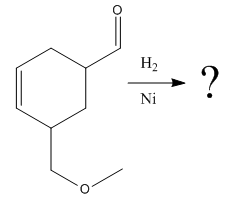
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one C=C bond and another the aldehyde group.
Aldehydes and alkenes have similar reactivity toward catalytic hydrogenation, but the aldehyde group in the above substrate is less sterically hindered; thus
Therefore, the product of the given reaction is
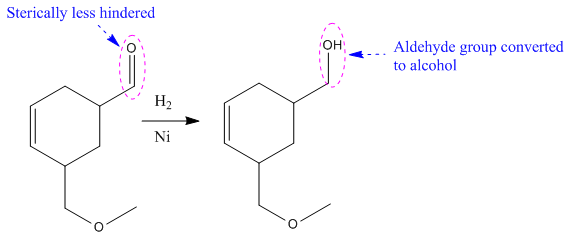
The product of the given reaction is predicted using the given reaction conditions.
(g)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
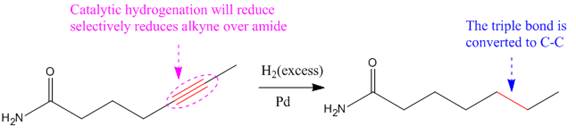
Explanation of Solution
The given reaction is
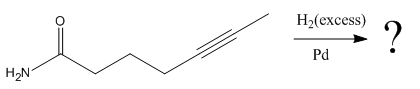
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one
The functional groups in alkenes, alkynes, and aldehydes can be selectively reduced over those in ketones, nitriles, and amides. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
Want to see more full solutions like this?
Chapter 19 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
- Question 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forwardQuestion 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forwardA. Add Water to below compound which 2-methyl 2-butene (addition Reaction) H₂C CH₂ CH, + H₂O-> ? Major product? Minor product? B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + Br₂→ ? Major product and Minor product both are same in this? C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition Reaction) H,C CH₂ CH₂ + HBr Major product? Minor product? D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + H₂ Major product and Minor product both are same in this?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





