
(a)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Alkylation of carbonyl compound can take place at the α-carbon via nucleophilic substitution reaction. In this reaction acidic proton of the carbonyl compound is abstracted by the strong base an enolate anion is formed. The attack of this nucleophile takes place at the electrophilic carbon of the alkyl bromide and bromine atom is displaced through the
Answer to Problem 19.73P
The given synthesis is carried out as:
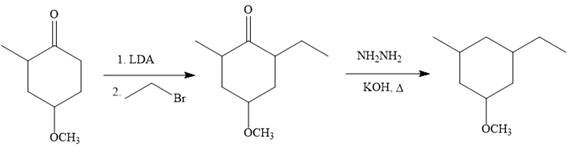
Explanation of Solution
The given synthesis:
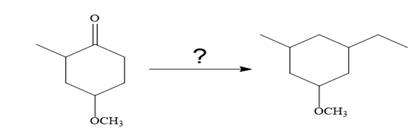
So the given synthesis is carried out below:
In the first step, the nucleophilic addition of the enolate anion to the ethyl bromide takes place shown below:
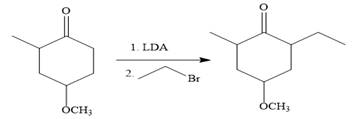
Note than LDA is sterically hindered strong base, therefore, abstract less sterically hindered proton selectively.
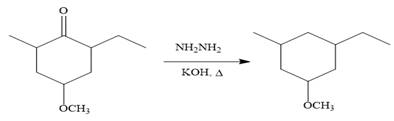
Finally, the carbonyl group is reduced to a corresponding methylene group (
It is shown how to carry out the given synthesis by using any reagents necessary.
(b)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Alkylation of carbonyl compound can take place at the α carbon via nucleophilic substitution reaction. In this reaction acidic proton of the carbonyl compound is abstracted by the strong base an enolate anion is formed. The attack of this nucleophile takes place at the electrophilic carbon of the alkyl bromide and bromine atom is displaced through
Answer to Problem 19.73P
The given synthesis is carried out as:
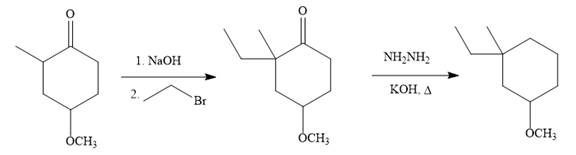
Explanation of Solution
The given synthesis is
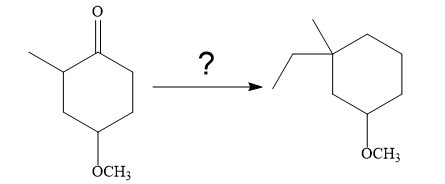
So the given synthesis is carried out below:
In the first step, the nucleophilic addition of the enolate anion to the ethyl bromide takes place shown below:
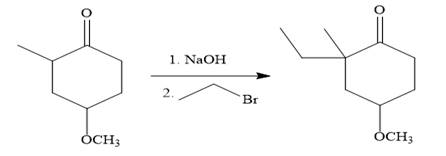
The require nucleophile is generated by the treatment of the strong base
Finally, the carbonyl group is reduced to a corresponding methylene group (
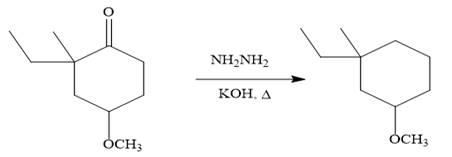
It is shown how to carry out the given synthesis by using any reagents necessary.
(c)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Lithium dialkylcuprate,
Answer to Problem 19.73P
The given synthesis is carried out as:
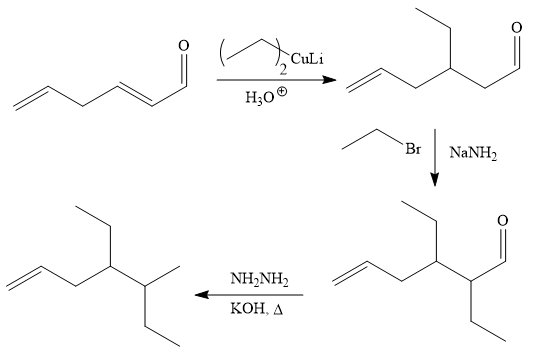
Explanation of Solution
The given synthesis is
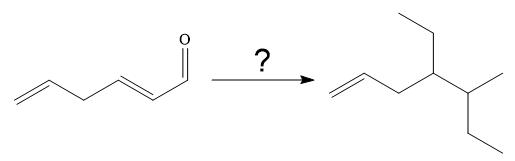
It is noticed that one ethyl group is added to the 3-position of the original compound, therefore, the conjugate addition must be needed.
So the given synthesis is carried out below:
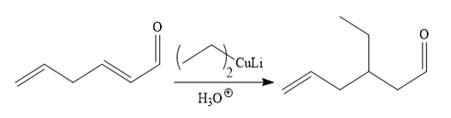
The first conjugate addition is carried out by the treatment of lithium diethylcuprate, a Gilman reagent with the given starting material. Since the conjugate addition is irreversible to the carbonyl group and regenerated during the workup step.
The above product then treated with ethyl bromide in the presence of a strong base
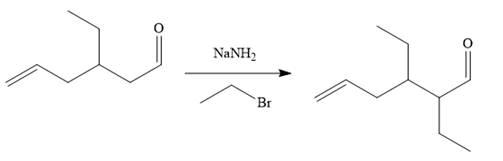
Finally, the carbonyl group is reduced to a corresponding methyl group (
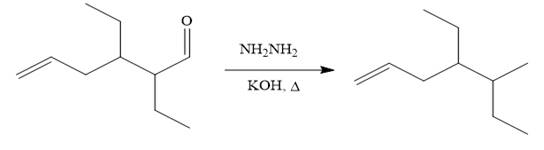
It is shown how to carry out the given synthesis by using any reagents necessary.
(d)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
LDA is the strong non-nucleophilic base, due to bulky isopropyl groups. Therefore, it abstract proton of less sterically hindered α-carbon of the carbonyl compound. The reduction of the carbonyl group of a ketone to a methylene (
Answer to Problem 19.73P
The given synthesis is carried out as:
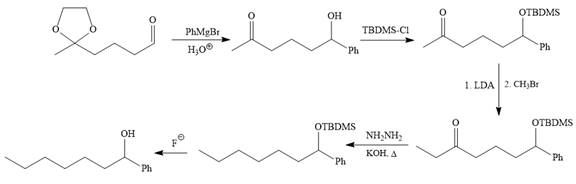
Explanation of Solution
The given synthesis is
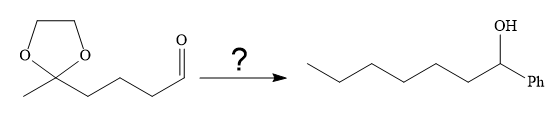
It is noticed that the phenyl group is added to the carbonyl carbon of the given starting material. So Grignard reaction carried out first as below:
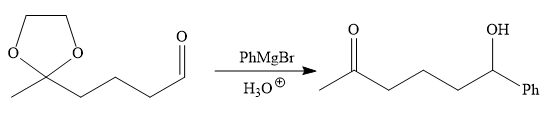
Since acidic condition required in workup step of Grignard reaction, the protected carbonyl group deprotected automatically because in acidic condition acetal protection also deprotect and the carbonyl group regenerated (right to the above product).
The alkylation of right carbon of the regenerated carbonyl compound is needed and it is done by using the sterically hindered base, LDA followed by methyl bromide. The bromine atom is replaced by a nucleophile (an enolate anion) via
The substrate is first treated with
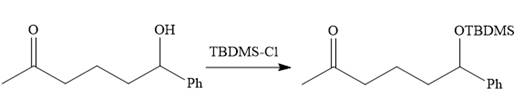
Now the required alkylation is carried out as below:
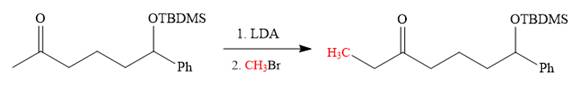
Finally, the carbonyl group is reduced to a corresponding methylene group (
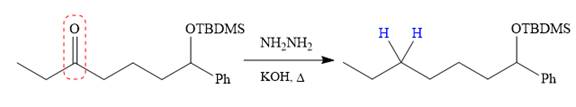
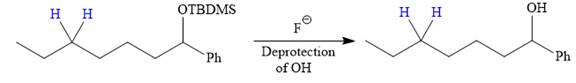
The protected OH in the form of OTBDMS is deprotected by the use of the source of
It is shown how to carry out the given synthesis by using any reagents necessary.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry: Principles And Mechanisms
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





