
Concept explainers
(a)
Interpretation:
The
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is
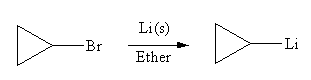
Explanation of Solution
The given reaction is,
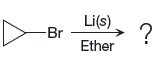
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:
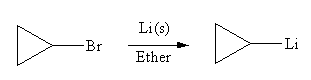
The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(b)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF).
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
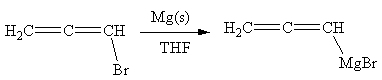
Explanation of Solution
The given reaction is
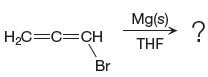
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF). So the C-Br bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(c)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF).
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
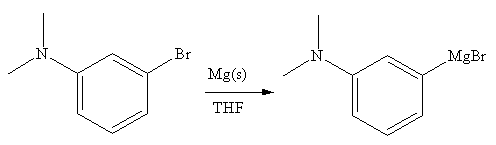
Explanation of Solution
The given reaction is
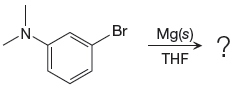
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF). So the C-Br bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(d)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgX) simply by treating it with solid magnesium in an ether solvent.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
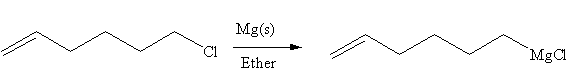
Explanation of Solution
The given reaction is
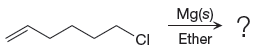
In the above reaction, the C atom of the C-Cl bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl chloride can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether. So the C-Cl bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:
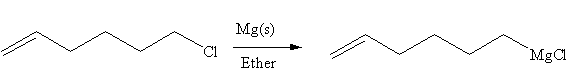
The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(e)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
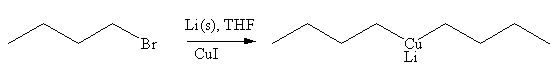
Explanation of Solution
The given reaction is
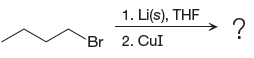
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. Lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI. So the C-Br bond will become C-CuLi bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(f)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether solvent such as THF.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
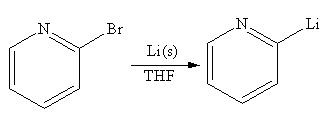
Explanation of Solution
The given reaction is
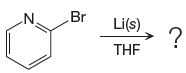
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether solvent such as THF. So the C-Br bond will become C-Li bond. Therefore the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(g)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
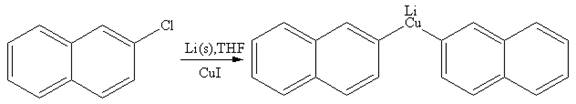
Explanation of Solution
The given reaction is
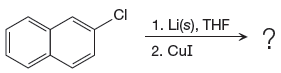
In the above reaction, the C atom of the C-Cl bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



