
Concept explainers
a)
Interpretation:
Half reactions; the anode and cathode have to be labelled.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
The change in free-energy represents the maximum amount of useful work that can be obtained in a reaction:
Relation between
Relation between
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
a)
Explanation of Solution
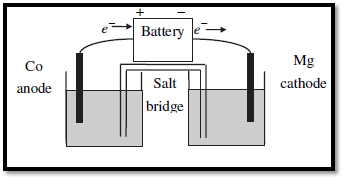
Figure.1
For the given redox reactions,
In the galvanic cell, Oxidation occurs at anode and reduction occurs at cathode.
Therefore,
Anode (Oxidation):
Cathode (Reduction):
b)
Interpretation:
The minimum voltage needed to drive the reaction has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
b)
Explanation of Solution
The emf values for the two given half-reactions are,
Anode (Oxidation):
Cathode (Reduction):
Calculated standard emf for galvanic cell as follows,
The minimum voltage needed to drive the reaction is
c)
Interpretation:
The emf of a given galvanic cell has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
c)
Explanation of Solution
Given: Current= 10.0 A; Time,
Convert Current into coulomb:
As known,
Convert number of Coulombs into mole of electrons:
Convert mole of electrons into number of moles:
1 mole of Cobalt
‘X’of Cobalt =
Therefore, no.of moles of Cobalt is
Assuming solution volumes of 1.00L, the concentration of Co2+ in solution after 2hours is 2.373 M, and the concentration of Mg2+ in solution after 2 hours is 1.627 M. we use the Nernst equation to solve for
Calculation of non-standard emf value using Nernst equation:
The reaction quotient for the given reaction is,
The concentration of pure solids and pure liquids do not appear in the expression for Q.
Hence, the reaction quotient becomes,
Substitute known constant values of R, T and F into Nernst equation becomes as follows,
The number of electrons transferred in the given redox reaction is TWO (n=2) and
The emf of the given cell reaction is
Want to see more full solutions like this?
Chapter 19 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
- Manoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forwardIn the table below, the correct structure for (2R)-3-methylpentan-2-ol (IUPAC name) can be represented by the letter OH OH HE > ' ÕH C B OH D A/ E OHarrow_forwardPredict the major products of the following organic reaction: + A Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Check Click and drag to start drawing a structure. Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





