
Fundamentals of Physics, Volume 1, Chapter 1-20
10th Edition
ISBN: 9781118233764
Author: David Halliday
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 15P
Suppose 0.825 mol of an ideal gas undergoes an isothermal expansion as energy is added to it as heat Q. If Fig. 19-21 shows the final volume Vf versus Q, what is the gas temperature? The stale of the vertical axis is set by Vfs = 0.30 m3, and the scale of the horizontal axis is set by Qs = 1200 J.
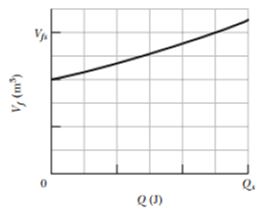
Figure 19-21 Problem 15.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lab 8 Part 3 PHET Wave Interface simulation.
I am having trouble with this part of the lab.
Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?
Hi,
I have canceled, why did you charge me again?
Chapter 19 Solutions
Fundamentals of Physics, Volume 1, Chapter 1-20
Ch. 19 - For four situations for an ideal gas, the table...Ch. 19 - In the p-V diagram of Fig. 19-17, the gas does 5 J...Ch. 19 - For a temperature increase of T1, a certain amount...Ch. 19 - The dot in Fig, 19-18a represents the initial...Ch. 19 - A certain amount of energy is to be transferred as...Ch. 19 - The dot in Fig. 19-18b represents the initial...Ch. 19 - a Rank the four paths of Fig. 19-16 according to...Ch. 19 - The dot in Fig. 19-18c represents the initial...Ch. 19 - Prob. 9QCh. 19 - Does the temperature of an ideal gas increase,...
Ch. 19 - Prob. 1PCh. 19 - Gold has a molar mass of 197 g/mol. a How many...Ch. 19 - SSM Oxygen gas having a volume of 1000 cm3 at...Ch. 19 - A quantity of ideal gas at: 10.0C and 100 kPa...Ch. 19 - The best laboratory vacuum has a pressure of about...Ch. 19 - Water bottle in a hot car. In the American...Ch. 19 - Suppose 1.80 mol of an ideal gas is taken from a...Ch. 19 - Compute a the number of moles and b the number of...Ch. 19 - An automobile tire has a volume of 1.64 102 m3...Ch. 19 - A container encloses 2 mol of an ideal gas that...Ch. 19 - SSM ILW WWW Air that initially occupies 0.140 m3...Ch. 19 - GO Submarine rescue. When the U.S. submarine...Ch. 19 - Prob. 13PCh. 19 - In the temperature range 310 K to 330 K, the...Ch. 19 - Suppose 0.825 mol of an ideal gas undergoes an...Ch. 19 - An air bubble of volume 20 cm3 is at the bottom of...Ch. 19 - GO Container A in Fig. 19-22 holds an ideal gas at...Ch. 19 - The temperature and pressure in the Suns...Ch. 19 - a Compute the rms speed of a nitrogen molecule at...Ch. 19 - Calculate the rms speed of helium atoms at 1000 K....Ch. 19 - SSM The lowest possible temperature in outer space...Ch. 19 - Find the rms speed of argon atoms at 313 K. See...Ch. 19 - A beam of hydrogen molecules H2 is directed toward...Ch. 19 - At 273 K and 1.00 102 atm, the density of a gas...Ch. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Water standing in the open at 32.0C evaporates...Ch. 19 - At what frequency would the wavelength of sound in...Ch. 19 - SSM The atmospheric density at an altitude of 2500...Ch. 19 - Prob. 30PCh. 19 - In a certain particle accelerator, protons travel...Ch. 19 - Prob. 32PCh. 19 - Prob. 33PCh. 19 - Prob. 34PCh. 19 - Prob. 35PCh. 19 - The most probable speed of the molecules in a gas...Ch. 19 - Prob. 37PCh. 19 - Figure 19-24 gives the probability distribution...Ch. 19 - At what temperature does the rms speed of a...Ch. 19 - Two containers are at the same temperature. The...Ch. 19 - Prob. 41PCh. 19 - What is the internal energy of 1.0 mol of an ideal...Ch. 19 - Prob. 43PCh. 19 - GO One mole of ail ideal diatomic gas goes from a...Ch. 19 - ILW The mass of a gas molecule can be computed...Ch. 19 - Under constant pressure, the temperature of 2.00...Ch. 19 - The temperature of 2.00 mol of an ideal monatomic...Ch. 19 - GO When 20.9 J was added as heat to a particular...Ch. 19 - SSM A container holds a mixture of three...Ch. 19 - We give 70 J as heat to a diatomic gas, which then...Ch. 19 - Prob. 51PCh. 19 - GO Suppose 12.0 g of oxygen O2 gas is heated at...Ch. 19 - SSM WWW Suppose 4.00 mol of an ideal diatomic gas...Ch. 19 - We know that for an adiabatic process pV = a...Ch. 19 - A certain gas occupies a volume of 4.3 L at a...Ch. 19 - Suppose 1.00 L of a gas with = 1.30, initially at...Ch. 19 - The volume of an ideal gas is adiabatically...Ch. 19 - GO Opening champagne. In a bottle of champagne,...Ch. 19 - GO Figure 19-26 shows two paths that may be taken...Ch. 19 - GO Adiabatic wind. The normal airflow over the...Ch. 19 - GO A gas is to be expanded from initial state i to...Ch. 19 - GO An ideal diatomic gas, with rotation but no...Ch. 19 - Figure 19-27 shows a cycle undergone by 1.00 mol...Ch. 19 - Calculate the work done by an external agent...Ch. 19 - An ideal gas undergoes an adiabatic compression...Ch. 19 - Prob. 66PCh. 19 - An ideal monatomic gas initially has a temperature...Ch. 19 - Prob. 68PCh. 19 - SSM The envelope and basket of a hot-air balloon...Ch. 19 - An ideal gas, at initial temperature T1 and...Ch. 19 - Prob. 71PCh. 19 - At what temperature do atoms of helium gas have...Ch. 19 - Prob. 73PCh. 19 - Prob. 74PCh. 19 - The temperature of 3.00 mol of a gas with CV =...Ch. 19 - During a compression at a constant pressure of 250...Ch. 19 - SSM Figure 19-28 shows a hypothetical speed...Ch. 19 - Prob. 78PCh. 19 - SSM An ideal gas undergoes isothermal compression...Ch. 19 - Oxygen O2 gas at 273 K and 1.0 atm is confined to...Ch. 19 - An ideal pas is taken through a complete cycle in...Ch. 19 - Prob. 82PCh. 19 - SSM A sample of ideal gas expands from an initial...Ch. 19 - An ideal gas with 3.00 mol is initially in state 1...Ch. 19 - A steel lank contains 300 g of ammonia gas NH3 at...Ch. 19 - In an industrial process the volume of 25.0 mol of...Ch. 19 - Figure 19-29 shows a cycle consisting of five...Ch. 19 - An ideal gas initially at 300 K is compressed at a...Ch. 19 - A pipe of length L = 25.0 m that is open at one...Ch. 19 - In a motorcycle engine, a piston is forced down...Ch. 19 - For adiabatic processes in an ideal gas, show that...Ch. 19 - Air at 0.000C and 1.00 atm pressure has a density...Ch. 19 - Prob. 93PCh. 19 - Prob. 94PCh. 19 - Prob. 95PCh. 19 - For air near 0C, by how much does the speed of...Ch. 19 - Prob. 97P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which of the roll owing compounds have a dipole moment of zero?
Organic Chemistry (8th Edition)
Did all the organisms living in or on the environments sampled grow on your nutrient agar? Briefly explain.
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
The method of comparison of different fuels is to be described. Concept introduction: Combustion reactions are ...
Living By Chemistry: First Edition Textbook
What are the two types of bone marrow, and what are their functions?
Human Anatomy & Physiology (2nd Edition)
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective (8th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forwardA planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forward
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY