
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 74GP
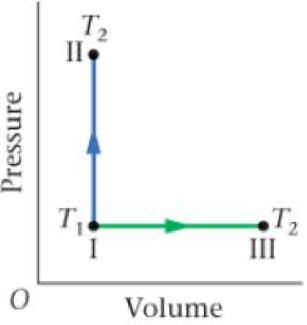
Figure 18-34 Problem 74
74 • CE An ideal gas has the pressure and volume indicated by point I in Figure 18-34. At this point its temperature is T1. The temperature of the gas can be increased to T2 by using the constant-volume process, I → II, or the constant-pressure process, I → III. Is the entropy change for the process I → II greater than, less than, or equal to the entropy change on the process I → III? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
No chatgpt pls will upvote
No chatgpt pls will upvote
Chapter 18 Solutions
Physics (5th Edition)
Ch. 18.1 - System 1 is at 0 C and system 2 is at 0 F. If...Ch. 18.2 - Enhance Your Understanding (Answers given at the...Ch. 18.3 - Enhance Your Understanding (Answers given at the...Ch. 18.4 - Enhance Your Understanding (Answers given at the...Ch. 18.5 - Enhance Your Understanding (Answers given at the...Ch. 18.6 - Enhance Your Understanding (Answers given at the...Ch. 18.7 - Enhance Your Understanding (Answers given at the...Ch. 18.8 - Enhance Your Understanding (Answers given at the...Ch. 18.9 - Enhance Your Understanding (Answers given at the...Ch. 18.10 - Enhance Your Understanding (Answer given at the...
Ch. 18 - Prob. 1CQCh. 18 - Heat is added to a substance. Is it safe to...Ch. 18 - Are there thermodynamic processes in which all the...Ch. 18 - An ideal gas is held in an insulated container at...Ch. 18 - Prob. 5CQCh. 18 - Which law of thermodynamics would be violated if...Ch. 18 - Heat engines always give off a certain amount of...Ch. 18 - Prob. 8CQCh. 18 - Which law of thermodynamics is most pertinent to...Ch. 18 - Which has more entropy: (a) popcorn kernels, or...Ch. 18 - Prob. 1PCECh. 18 - A gas expands, doing 100 J of work. How much heat...Ch. 18 - A swimmer does 7.7 105 J of work and gives off...Ch. 18 - When 1310 J of heat are added to one mole of an...Ch. 18 - Three different processes act on a system. (a) In...Ch. 18 - A container holds a gas consisting of 2.85 moles...Ch. 18 - The Charge on Adhesive Tape When adhesive tape is...Ch. 18 - Predict/Calculate One mole of an ideal monatomic...Ch. 18 - Prob. 9PCECh. 18 - A cylinder contains 4.0 moles of a monatomic gas...Ch. 18 - An ideal gas is taken through the three processes...Ch. 18 - Figure 18-26 shows three different multistep...Ch. 18 - Prob. 13PCECh. 18 - An ideal gas is compressed at constant pressure to...Ch. 18 - As an ideal gas expands at constant pressure from...Ch. 18 - A system consisting of an ideal gas at the...Ch. 18 - Prob. 17PCECh. 18 - (a) Find the work done by a monatomic ideal gas as...Ch. 18 - Prob. 19PCECh. 18 - Predict/Calculate If 9.50 moles of a monatomic...Ch. 18 - Suppose 118 moles of a monatomic ideal gas undergo...Ch. 18 - A weather balloon contains an ideal gas and has a...Ch. 18 - Prob. 23PCECh. 18 - During an adiabatic process, the temperature of...Ch. 18 - An ideal gas follows the three-part process shown...Ch. 18 - With the pressure held constant at 260 kPa, 43 mol...Ch. 18 - Prob. 27PCECh. 18 - A system expands by 0.75 m3 at a constant pressure...Ch. 18 - Prob. 29PCECh. 18 - A certain amount of a monatomic ideal gas...Ch. 18 - An ideal gas doubles its volume in one of three...Ch. 18 - Predict/Explain You plan to add a certain amount...Ch. 18 - Find the amount of heat needed to increase the...Ch. 18 - (a) If 585 J of heat are added to 49 moles of a...Ch. 18 - A system consists of 3.5 mol of an ideal monatomic...Ch. 18 - Find the change in temperature if 170 J of heat...Ch. 18 - Gasoline Ignition Consider a short time span just...Ch. 18 - Prob. 38PCECh. 18 - Prob. 39PCECh. 18 - A monatomic ideal gas is held in a thermally...Ch. 18 - Consider the expansion of 60.0 moles of a...Ch. 18 - A Carnot engine can be operated with one of the...Ch. 18 - What is the efficiency of an engine that exhausts...Ch. 18 - An engine receives 660 J of heat from a hot...Ch. 18 - A Carnot engine operates between the temperatures...Ch. 18 - A nuclear power plant has a reactor that produces...Ch. 18 - At a coal-burning power plant a steam turbine is...Ch. 18 - Predict/Calculate A portable generator produces...Ch. 18 - Predict/Calculate The efficiency of a particular...Ch. 18 - During each cycle a reversible engine absorbs 3100...Ch. 18 - Prob. 51PCECh. 18 - The operating temperatures for a Carnot engine are...Ch. 18 - A certain Carnot engine takes in the heat Qh and...Ch. 18 - Predict/Explain (a) If the temperature in the...Ch. 18 - The refrigerator in your kitchen does 490 J of...Ch. 18 - A refrigerator with a coefficient of performance...Ch. 18 - Prob. 57PCECh. 18 - Prob. 58PCECh. 18 - An air conditioner is used to keep the interior of...Ch. 18 - A reversible refrigerator has a coefficient of...Ch. 18 - A freezer has a coefficient of performance equal...Ch. 18 - Predict/Explain (a) If you rub your hands...Ch. 18 - Predict/Explain (a) An ideal gas is expanded...Ch. 18 - Predict/Explain (a) A gas is expanded reversibly...Ch. 18 - Find the change in entropy when 1.85 kg of water...Ch. 18 - Determine the change in entropy that occurs when...Ch. 18 - Prob. 67PCECh. 18 - On a cold winters day heat leaks slowly out of a...Ch. 18 - An 88-kg parachutist descends through a vertical...Ch. 18 - Predict/Calculate Consider the air-conditioning...Ch. 18 - A heat engine operates between a high-temperature...Ch. 18 - It can be shown that as a mass m with specific...Ch. 18 - Prob. 73GPCh. 18 - Figure 18-34 Problem 74 74 CE An ideal gas has...Ch. 18 - The heat that goes into a particular Carnot engine...Ch. 18 - Predict/Calculate Consider 132 moles of a...Ch. 18 - Prob. 77GPCh. 18 - Prob. 78GPCh. 18 - Predict/Calculate Engine A has an efficiency of...Ch. 18 - Nuclear Versus Natural Gas Energy Because of...Ch. 18 - A freezer with a coefficient of performance of...Ch. 18 - Entropy and the Sun The surface of the Sun has a...Ch. 18 - Prob. 83GPCh. 18 - A cylinder with a movable piston holds 2.95 mol of...Ch. 18 - Making Ice You place 0.410 kg of cold water inside...Ch. 18 - An inventor claims a new cyclic engine that uses...Ch. 18 - Predict/Calculate A small dish containing 530 g of...Ch. 18 - Predict/Calculate An ideal gas is taken through...Ch. 18 - One mole of an ideal monatomic gas follows the...Ch. 18 - When a heat Q is added to a monatomic ideal gas at...Ch. 18 - The Carnot Cycle Figure 18-36 shows an example of...Ch. 18 - A Carnot engine and a Carnot refrigerator operate...Ch. 18 - Prob. 93PPCh. 18 - Energy from the Ocean Whenever two objects are at...Ch. 18 - Prob. 95PPCh. 18 - Energy from me Ocean Whenever two objects are at...Ch. 18 - Predict/Calculate Referring to Example 18-21...Ch. 18 - Predict/Calculate Referring to Example 18-21...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (7th Edition)
28. Consider the reaction
Express the rate of the reaction in terms of the change in concentration of e...
Chemistry: Structure and Properties (2nd Edition)
3. Current Iin flows into three resistors connected together one after the other as shown in Figure Q23.3. The ...
College Physics: A Strategic Approach (3rd Edition)
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
3. What are serous membranes, and what are their functions?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forwardA planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forward
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
- Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY