
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 45E
Consider the following galvanic cells:
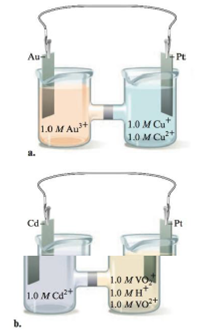
For each galvanic cell, give the balanced cell equation and determine 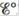 . Standard reduction potentials are found in Table 17-1.
. Standard reduction potentials are found in Table 17-1.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
11. Complete the following esterification reaction with names of all the reactants and products under.
Hint: Remove the water and end up with ester
R-C-OH + ROH
R-C-OR + H₂O
A carboxylic acid
An alcohol
An ester
Water
BYJU'S
H-C-C
O-H
Нин
C-C-C-H
HAAA
H O-C-C-C-H
AAA
Ethanoic acid
Propanol
Water Propyl ethanoate
By com
CH3COOH + CH3CH2CH2CH₂CH₂OH →
Practice for alcohols aldehydes and ketones:
12. Draw the structures from the following names mixed of alcohol/aldehyde and ketone:
a. 4-methyl cyclohexanone
b. 3-methyl-2-pentenal
c. 2,3-dimethylcyclohexanone
d. 1,3propanediol or Propane 1,3 diol
13. Write systematic names for the following compounds identify
functional group:
a.
b. (CH3)2CH-C
OH
c) CH(CH₂)--
OH
-,-,
may you please show all steps! i am having a hard time understanding and applying in this format, thank you!
10. Complete the substitution reaction of 2 pentanol with these reagents.
Reagents & Reaction Conditions use practice sheet. Please write only
major products, minor product like water, other gases are not
required.
Hint: In substitution of alcohol, we generally substitute OH group
with Halogens like cl, Br, F using some reagent containing
halogens. Ensure to add halogens to the same carbon number
where you are removing OH from
Examples
Alcohols can be converted to Alkyl Halides with HX acids
HBr
H₂O
HCI
+ H₂O
HI
+
H₂O
CH,CH₂OH + SOCI₂
CH,CH₂OH + PCI₁₂
A
BBYJU'S
CH CHCI + SO₂+ HCI
CH₂CH CIP(OH), + HCI
CH,CH₂OH + PCI CHCHCI + POCI + HCI
CH,CH₂OH + PBr, CH,CH,Br + P(OH), + HBr
1. Reaction with HBr with 2 Pentanol
2.Reaction with HI with 2 pentanol
© Byjus.com
3.Reaction with HCI+ZnCl,, with 2 pentanol (Zncl2 is catalyst no role)
4.Reaction with SOCI,, with 2 Pentanol
5.Reaction with PBr; or PCl, with 2 pentanol
Chapter 18 Solutions
Chemistry
Ch. 18 - What is a half-reaction? Why must the number of...Ch. 18 - Galvanic cells harness spontaneous...Ch. 18 - Table 17-1 lists common half-reactions along with...Ch. 18 - Consider the equation G = -nF. What are the four...Ch. 18 - The Nernst equation allows determination of the...Ch. 18 - What are concentration cells? What is in a...Ch. 18 - Prob. 7RQCh. 18 - Prob. 8RQCh. 18 - What characterizes an electrolytic cell? What is...Ch. 18 - Prob. 1ALQ
Ch. 18 - When balancing reactions in Chapter 3, we did not...Ch. 18 - Sketch a galvanic cell, and explain how it works....Ch. 18 - In making a specific galvanic cell, explain how...Ch. 18 - Prob. 5ALQCh. 18 - Prob. 6ALQCh. 18 - Sketch a cell that forms iron metal from iron(II)...Ch. 18 - Which of the following is the best reducing agent:...Ch. 18 - You are told that metal A is a better reducing...Ch. 18 - Explain the following relationships: G and w, cell...Ch. 18 - Explain why cell potentials are not multiplied by...Ch. 18 - What is the difference between and ? When is equal...Ch. 18 - Consider the following galvanic cell: What happens...Ch. 18 - Look up the reduction potential for Fe3+ to Fe2+....Ch. 18 - If the cell potential is proportional to work and...Ch. 18 - Is the following statement true or false?...Ch. 18 - Define oxidation and reduction in terms of both...Ch. 18 - Assign oxidation numbers to all the atoms in each...Ch. 18 - Specify which of the following equations represent...Ch. 18 - The Ostwald process for the commercial production...Ch. 18 - Balance the following oxidation-reduction...Ch. 18 - Balance the following oxidation-reduction...Ch. 18 - What is electrochemistry? What are redox...Ch. 18 - Prob. 24QCh. 18 - When magnesium metal is added to a beaker of...Ch. 18 - How can one construct a galvanic cell from two...Ch. 18 - The free energy change for a reaction, G, is an...Ch. 18 - What is wrong with the following statement: The...Ch. 18 - When jump-starting a car with a dead battery, the...Ch. 18 - In theory, most metals should easily corrode in...Ch. 18 - Consider the electrolysis of a molten salt of some...Ch. 18 - Consider the following electrochemical cell: a. If...Ch. 18 - Prob. 33QCh. 18 - Prob. 34QCh. 18 - Consider the following galvanic cell: Label the...Ch. 18 - Consider the following galvanic cell: a. Label the...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Consider the following galvanic cells: For each...Ch. 18 - Give the balanced cell equation and determine for...Ch. 18 - Calculate values for the following g cells. Which...Ch. 18 - Calculate values for the following cells. Which...Ch. 18 - Chlorine dioxide (C1O2), which is produced by the...Ch. 18 - The amount of manganese in steel is determined by...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Estimate for the half-reaction 2H2O+2eH2+2OH given...Ch. 18 - The equation G = nF also can be applied to...Ch. 18 - Glucose is the major fuel for most living cells....Ch. 18 - Direct methanol fuel cells (DMFCs) have shown some...Ch. 18 - Using data from Table 17-1, place the following in...Ch. 18 - Using data from Table 17-1, place the following in...Ch. 18 - Answer the following questions using data from...Ch. 18 - Answer the following questions using data from...Ch. 18 - Consider only the species (at standard conditions)...Ch. 18 - Prob. 62ECh. 18 - Use the table of standard reduction potentials...Ch. 18 - Consider the concentration cell in Fig. 17-10. If...Ch. 18 - Consider the concentration cell shown below....Ch. 18 - Consider a concentration cell similar to the one...Ch. 18 - The overall reaction in the lead storage battery...Ch. 18 - Calculate the pH of the cathode compartment for...Ch. 18 - Consider the cell described below:...Ch. 18 - Consider the cell described below:...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a standard...Ch. 18 - Prob. 78ECh. 18 - An electrochemical cell consists of a standard...Ch. 18 - An electrochemical cell consists of a nickel metal...Ch. 18 - Consider a concentration cell that has both...Ch. 18 - You have a concentration cell in which the cathode...Ch. 18 - Under standard conditions, what reaction occurs,...Ch. 18 - A disproportionation reaction involves a substance...Ch. 18 - Consider the following galvanic cell at 25C:...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - Cadmium sulfide is used in some semiconductor...Ch. 18 - For the following half-reaction, = 2.07 V:...Ch. 18 - Calculate for the following half-reaction:...Ch. 18 - The solubility product for CuI(s) is 1.1 102...Ch. 18 - How long will it take to plate out each of the...Ch. 18 - The electrolysis of BiO+ produces pure bismuth....Ch. 18 - What mass of each of the following substances can...Ch. 18 - Aluminum is produced commercially by the...Ch. 18 - Electrolysis of an alkaline earth metal chloride...Ch. 18 - What volume of F2 gas, at 25C and 1.00 atm, is...Ch. 18 - What volumes of H2(g) and O2(g) at STP are...Ch. 18 - A single HallHeroult cell (as shown in Fig. 17-22)...Ch. 18 - A factory wants to produce 1.00 103 kg barium...Ch. 18 - It took 2.30 min using a current of 2.00 A to...Ch. 18 - A solution containing Pt4+ is electrolyzed with a...Ch. 18 - A solution at 25C contains 1.0 M Cd2+, 1.0 M Ag+,...Ch. 18 - A solution at 25C contains 1.0 M Cu2 and 1.0 104...Ch. 18 - In the electrolysis of an aqueous solution of...Ch. 18 - Copper can be plated onto a spoon by placing the...Ch. 18 - Prob. 107ECh. 18 - Prob. 108ECh. 18 - What reactions take place at the cathode and the...Ch. 18 - What reaction will take place at the Cathode and...Ch. 18 - The saturated calomel electrode. abbreviated SCE....Ch. 18 - Consider the following half-reactions: Explain why...Ch. 18 - Consider the standard galvanic cell based on the...Ch. 18 - A standard galvanic cell is constructed so that...Ch. 18 - The black silver sulfide discoloration of...Ch. 18 - Prob. 116AECh. 18 - When aluminum foil is placed in hydrochloric acid,...Ch. 18 - Prob. 118AECh. 18 - Prob. 119AECh. 18 - Prob. 120AECh. 18 - A fuel cell designed to react grain alcohol with...Ch. 18 - The overall reaction and equilibrium constant...Ch. 18 - Prob. 123AECh. 18 - The overall reaction and standard cell potential...Ch. 18 - Prob. 125AECh. 18 - The ultimate electron acceptor in the respiration...Ch. 18 - One of the few industrial-scale processes that...Ch. 18 - It took 150. s for a current of 1.25 A to plate...Ch. 18 - Prob. 129AECh. 18 - In the electrolysis of a sodium chloride solution,...Ch. 18 - An aqueous solution of an unknown salt of...Ch. 18 - Which of the following statement(s) is/are true?...Ch. 18 - Consider a galvanic cell based on the following...Ch. 18 - Prob. 134CWPCh. 18 - Consider a galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - An aqueous solution of PdCl2 is electrolyzed for...Ch. 18 - Consider the following half-reactions:...Ch. 18 - Consider the following reduction potentials: Co3++...Ch. 18 - Calculate and G for the reaction 2H2O(l) 2H2(g)...Ch. 18 - Prob. 141CPCh. 18 - The overall reaction in the lead storage battery...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - A zinc-copper battery is constructed at follows at...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Consider a cell based on the following...Ch. 18 - Prob. 147CPCh. 18 - You have a concentration cell with Cu electrodes...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Given the following two standard reduction...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - Prob. 152CPCh. 18 - Consider the following galvanic cell: A 15 0-mole...Ch. 18 - When copper reacts with nitric acid, a mixture of...Ch. 18 - The following standard reduction potentials have...Ch. 18 - An electrochemical cell is set up using the...Ch. 18 - Three electrochemical cells were connected in...Ch. 18 - A silver concentration cell is set up at 25C as...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - The table below lists the cell potentials for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does trandlation differ from transcription?
Microbiology: Principles and Explorations
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-methyl-2-propanol and also any oxidation products of 2- methyl-2- propanol. If there is more than one oxidation product, give the structure of each of the products. 4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a common name by which it is much better known (You'll see it in the grocery store or pharmacy). Give that common name 5. Aldehydes can be synthesized by the oxidation of. Please choose from below choices A. Primary alcohols B. Secondary alcohols C. Organic acids D. Inorganic acids 6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the product.arrow_forwardFinish the reactions hand written pleasearrow_forwardPart A Identify each alcohol as primary, secondary, or tertiary Drag the appropriate items to their respective bins. CH₂ H₂C- -C-OH HO CH₂ Primary Он OH CH₂ OH CCH₂OH CH₂ сн Secondary Tertiary Reset Help CH,CH₂ (CH)CHCH,OH CH,CH,CH,CCH, CHOH CH₂ Different types of alcohol groups Alcohol and its reaction: 8. Combing two alcohol molecules below and completing the reaction with Product .( Hint Reaction called etherification as ether is formed and name the ether once you complete the reaction. Hint.: R-O-H+H-O-RR-O-R Do the reaction: CH₂OH + CH₂OH---→ + H-O-H 9. Write the reaction of formation of alcohol from alkene by adding water: Addition reaction also called hydration reaction as we are adding water which occur always in presence of acid Hint: Break the double bond and add H and OH if symmetrical then add anywhere if unsymmetrical then follow Markovnikov rule H should go to that double bone carbon which has more hydrogen CH2=CH2 + H₂O-→arrow_forward
- Complete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forward
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY