
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 43P
Draw the products of each reaction.
a. 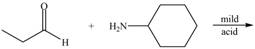 e.
e. 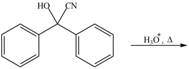
b. 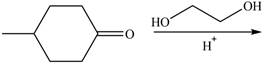 f.
f. 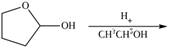
c. 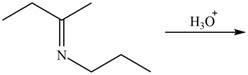 g.
g. 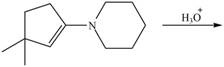
d. 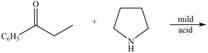 h.
h. 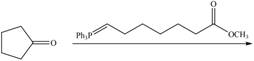
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Germanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap?
Group of answer choices
It is impossible to dope Ge and have this result in a larger bandgap.
Dope the Ge with silicon (Si)
Dope the Ge with gallium (Ga)
Dope the Ge with phosphorus (P)
Which of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band?
Group of answer choices
Si
Ge
InSb
CdS
Which of the following metals is the only one with all of its bands completely full?
Group of answer choices
K
Na
Ca
Al
Chapter 18 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 18.1 - Rank the following compounds in order of...Ch. 18.1 - Prob. 2PCh. 18.2 - Give the IUPAC name for each aldehyde.Ch. 18.2 - Prob. 4PCh. 18.2 - Give the IUPAC name for each ketone.Ch. 18.5 - Prob. 11PCh. 18.9 - Problem 21.17 Draw the products of the following...Ch. 18.9 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 18.9 - Problem 21.19 Draw the products (including...Ch. 18.9 - Problem 21.20 What starting materials are needed...
Ch. 18.9 - Prob. 19PCh. 18.10 - Problem 21.22 The product formed when reacts with...Ch. 18.10 - Prob. 21PCh. 18.11 - Prob. 22PCh. 18.11 - Prob. 23PCh. 18.11 - Prob. 24PCh. 18.12 - Prob. 25PCh. 18.12 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 18.13 - Problem 21.29 Draw the products of each...Ch. 18 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 18 - 21.41 Rank the following compounds in order of...Ch. 18 - Prob. 39PCh. 18 - 21.43 Give the IUPAC name for each compound.
a....Ch. 18 - 21.44 Give the structure corresponding to each...Ch. 18 - Prob. 42PCh. 18 - 21.46 Draw the products of each reaction.
a. e....Ch. 18 - Prob. 44PCh. 18 - 21.48 Draw all stereoisomers formed in each...Ch. 18 - Prob. 54PCh. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - Devise a synthesis of each alkene using a Wittig...Ch. 18 - Prob. 60PCh. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - 21.64 Draw a stepwise mechanism for the following...Ch. 18 - 21.65 Draw a stepwise mechanism f or the following...Ch. 18 - Prob. 67PCh. 18 - 21.67 Draw a stepwise mechanism for each...Ch. 18 - Prob. 69PCh. 18 - Prob. 70P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forwardFrom an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forward
- For which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forwardFor which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forward
- In the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forwardWhat is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forward
- PLEASE ANSWER THE QUESTION!!!arrow_forward3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forwardcurved arrows are used to illustate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction mechanism stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY