
(a)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 1. Identify and name the parent
- 2. Identify and name the substituent
- 3. Assign a locant to each substituent
- 4. Arrange the substituent alphabetically
(a)
Answer to Problem 28PP
- Name for the given compound
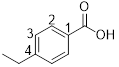
4-ethylbenzoic acid
Explanation of Solution
4-ethylbenzoic acid
From the structure of the compound it is clear that the parent group is benzoic acid.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number, for the given compound an ethyl group is a substituent and it is placed to the 4th (
(b)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 5. Identify and name the parent
- 6. Identify and name the substituent
- 7. Assign a locant to each substituent
- 8. Arrange the substituent alphabetically
(b)
Answer to Problem 28PP
- Name for the given compound
b)
-
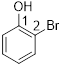
2-bromophenol
Explanation of Solution
The given compound b2-bromophenol
From the structure of the compound it is clear that the parent group is phenol.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound bromine atom is a substituent and they are placed to the 2nd (
(c)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 9. Identify and name the parent
- 10. Identify and name the substituent
- 11. Assign a locant to each substituent
- 12. Arrange the substituent alphabetically
(c)
Answer to Problem 28PP
- Name for the given compound
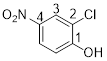
2-chloro-4-nitrophenol
Explanation of Solution
The given compound c
2-chloro-4-nitrophenol
From the structure of the compound it is clear that the parent group is phenol.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound a chlorine atom and nitro group are substituent and they are placed to the 2nd (
(d)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 13. Identify and name the parent
- 14. Identify and name the substituent
- 15. Assign a locant to each substituent
- 16. Arrange the substituent alphabetically
(d)
Answer to Problem 28PP
- Name for the given compound
d)
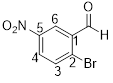
2-bromo-5-nitrobenzaldehyde
Explanation of Solution
The given compound d
2-bromo-5-nitrobenzaldehyde
From the structure of the compound it is clear that the parent group is benzaldehyde.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound a bromine atom and nitro group are substituent and they are placed to the 2nd (
(e)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 17. Identify and name the parent
- 18. Identify and name the substituent
- 19. Assign a locant to each substituent
- 20. Arrange the substituent alphabetically
(e)
Answer to Problem 28PP
- Name for the given compound
-
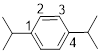
1, 4-diisopropylbenzene
Explanation of Solution
The given compound e
1, 4-diisopropylbenzene
From the structure of the compound it is clear that the parent group is benzene.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound there are two substituents, both isopropyl group it is placed to the 1st (
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry, Binder Ready Version
- Provide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardGiven a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forward
- The molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forwardIn GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forward
- How to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forwardGiven a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward
- 23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





