
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.8, Problem 24P
Convert each skeletal structure to a complete structure with all
a. 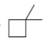 b.
b. 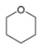 c.
c. 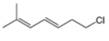 d.
d. 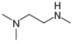
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please draw and explain
Describe each highlighted bond in terms of the overlap of atomic orbitals.
(a)
Н
Н
H
H
[References]
HIC H
H C H
H-C-CC-N:
H
σ character n character
(b)
HIC H
H H
H-C-C-C
HIC H
Н
H
O-H
σ character
n character
Submit Answer
Try Another Version
3 item attempts remaining
11
Naming and drawing alcohols
Write the systematic (IUPAC) name for each of the following organic molecules:
structure
OH
HO
OH
Explanation Check
name
☐
Chapter 1 Solutions
ORGANIC CHEMISTRY
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what is the drawn mechanism for diethyl carbonate and 4 - bromo - N, N -dimethylaniline to create crystal violet?arrow_forwardWhich of the following compounds are constitutional isomers of each other? I and II O II and III O III and IV OI and IV O II and IV CI H CI H CI H H CI H-C-C-CI C-C-C-CI H-C-C-CI H-C-C-CI H CI Ĥ ĆI A A Ĥ ĆI || IVarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Q1: Curved Arrows, Bronsted Acids & Bases, Lewis Acids & Bases Considering the following reactions: a) Predict the products to complete the reactions. b) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw some of the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. c) Label Bronsted acids and bases in the left side of the reactions. Label conjugate acids and bases in the right side of the reactions. d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. + OH CH30: OH B. + HBr C. H₂SO4 D. CF 3. CH 3 + HCI N H fluoxetine antidepressant 1↓ JDownloadarrow_forwardDon't used Ai solutionarrow_forwardPart 3: AHm,system Mass of 1.00 M HCI Vol. of 1.00 M HCI Mass of NaOH(s) Total Mass in Calorimeter Mole product if HCI limiting reactant Trial 1 62.4009 1.511g Mole product if NaOH limiting reactant Limiting reactant Initial Temperature Final Temperature 23.8°C 37.6°C Change in Temperature AHm,system (calculated) Average AHm,system (calculated) (calculated) (calculated) Trial 2 64.006g 1.9599 (calculated) (calculated) (calculated) (calculated) (calculated) (calculated) 24.7°C 41.9°C (calculated) (calculated) (2 pts. each)arrow_forward
- 1.) Using the graph below (including the line equation of y = -1.823x - 0.0162) What is the numerical value for the slope shown? 2.) What are the Unit(s) associated with the slope of the line shown? for we all remember that numerical data always has units. 3.) What would be a good title for this graph and explain your choice. 0.00 0.0 02 0.4 10.6 08 10 12 -0.20 -0.40 -0.60 -0.80 Temp, freezing, in degrees Celcius 5-1.00 -1.20 -1.40 -1:60 y=-1.823x-0.0162 -180 -2.00 Concentration of Sucrose (m)arrow_forwardDon't used Ai solutionarrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling. Please label in the image, so it fits explanation. I am still very unsure I undertand this.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License