
Concept explainers
(a)
To draw and name: The structure of α-keto acid which results from the transamination reaction of α-ketoglutarate with aspartate.
Introduction:
The α-amino acid is transferred to the a-carbon atom of α-ketoglutarate and leaves behind the corresponding α-keto acid is known as transamination reaction. This transfer of amino group is through the enzymes known as transferases or transaminases.
(a)
Answer to Problem 1P
Correct answer: Fig.1: Structure of oxaloacetate.
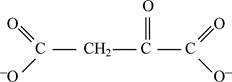
Fig.1: Structure of oxaloacetate.
Explanation of Solution
Explanation:
The structure of α-keto acid which results from the reaction of amino acids with α-ketoglutarate is as follow:
The final product obtained from the reaction is oxaloacetate.
(b)
To draw and name: The structure of α-keto acid which results from the transamination reaction of α-ketoglutarate with glutamate.
Introduction:
The α-amino acid is transferred to the a-carbon atom of α-ketoglutarate and leaves behind the corresponding α-keto acidis known as transamination reaction. This transfer of amino group is through the enzymes known as transferases or transaminases.
(b)
Answer to Problem 1P
Correct answer: Fig.1: Structure of α-ketoglutarate.
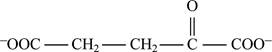
Fig.1: Structure of α-ketoglutarate.
Explanation of Solution
Explanation:
The structure of α-keto acid which results from the reaction of amino acids with α-ketoglutarate is as follow:
The α-ketoglutarate is the final product obtained from the reaction.
(c)
To draw and name: The structure of α-keto acid which results from the transamination reaction of α-ketoglutarate with alanine.
Introduction:
The α-amino acid is transferred to the a-carbon atom of α-ketoglutarate and leaves behind the corresponding α-keto acid is known as transamination reaction. This transfer of amino group is through the enzymes known as transferases or transaminases.
(c)
Answer to Problem 1P
Correct answer: Fig.1: Structure of pyruvate.
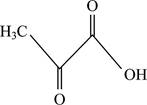
Fig.1: Structure of pyruvate.
Explanation of Solution
Explanation:
The structure of α-keto acid which results from the reaction of amino acids with α-ketoglutarate is as follow:
Pyruvate is the final product obtained from the reaction.
(d)
To draw and name: The structure of α-keto acid which results from the transamination reaction of α-ketoglutarate with phenylalnine.
Introduction:
The α-amino acid is transferred to the a-carbon atom of α-ketoglutarate and leaves behind the corresponding α-keto acid is known as transamination reaction. This transfer of amino group is through the enzymes known as transferases or transaminases.
(d)
Answer to Problem 1P
Correct answer: Fig.1: Structure of phenylpyruvate.
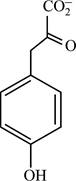
Fig.1: Structure of phenylpyruvate.
Explanation of Solution
Explanation:
The structure of α-keto acid which results from the reaction of amino acids with α-ketoglutarate is as follow:
phenylpyruvate is the final product obtained from the reaction.
Want to see more full solutions like this?
Chapter 18 Solutions
EBK LEHNINGER PRINCIPLES OF BIOCHEMISTR
- The following data were recorded for the enzyme catalyzed conversion of S -> P. Question: Estimate the Vmax and Km. What would be the rate at 2.5 and 5.0 x 10-5 M [S] ?arrow_forwardPlease helparrow_forwardThe following data were recorded for the enzyme catalyzed conversion of S -> P Question: what would the rate be at 5.0 x 10-5 M [S] and the enzyme concentration was doubled? Also, the rate given in the table is from product accumulation after 10 minuets of reaction time. Verify these rates represent a true initial rate (less than 5% turnover). Please helparrow_forward
- The following data was obtained on isocitrate lyase from an algal species. Identify the reaction catalyzed by this enzyme, deduce the KM and Vmax , and determine the nature of the inhibition by oxaloacetate. Please helparrow_forwardIn the table below, there are sketches of four crystals made of positively-charged cations and negatively-charged anions. Rank these crystals in decreasing order of stability (or equivalently increasing order of energy). That is, select "1" below the most stable (lowest energy) crystal. Select "2" below the next most stable (next lowest energy) crystal, and so forth. A B 鹽 (Choose one) +2 C +2 +2 (Choose one) D 鹽雞 (Choose one) (Choose one)arrow_forward1. Draw the structures for the fats A. 16:2: w-3 and B. 18:3:49,12,15 2. Name each of the molecules below (image attached)arrow_forward
- draw the structures for the fats A. 16:2:w-3 B 18:3:9,12,15arrow_forward1. Below is a template strand of DNA. Show the mRNA and protein that would result. label the ends of the molecules ( refer to attached image)arrow_forwardAttach the followina labels to the diagram below: helicase, single stranded binding proteins, lagging strand, leading strand, DNA polymerase, primase, 5' ends (3), 3' ends (3) (image attached)arrow_forward
- 1. How much energy in terms of ATP can be obtained from tristearin (stearate is 18:0) Show steps pleasearrow_forwardMultiple choice urgent!!arrow_forward1. Write the transamination reaction for alanine. Indicate what happens next to each of the molecules in the reaction, and under what conditions it happens. 2.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





