
Concept explainers
(a)
To determine: The type of reaction in which leucine is converted to α-ketoisocaproate.
Introduction:
The series of
β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(a)
Explanation of Solution
Pictorial representation: Fig. 1 represents the transamination reaction.
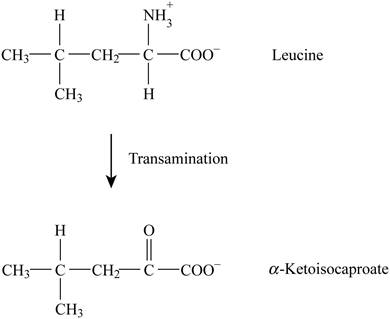
Fig. 1: Transamination reaction.
Explanation:
The transamination reaction is involved in the conversion of leucine into alpha ketoisocaproate. The co-factor involved in this reaction is PLP (pyridoxal phosphate). There is no analogous reaction to this reaction from citric acid cycle and β-oxidation.
(b)
To determine: The type of reaction in which Ketoisocaproate is converted to Isovaleryl-CoA.
Introduction:
The series of metabolic reactions, in which the energy stored in the compounds is released for the production of ATP (adenosine triphosphate), is called “Krebs’s cycle”. The pyruvate undergoes series of reactions in the citric acid cycle and converts into Acetyl CoA (acetyl coenzyme A) in the presence of oxygen. β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(b)
Explanation of Solution
Pictorial representation: Fig. 2 represents the oxidative decarboxylation.
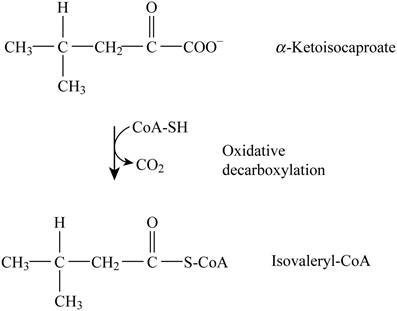
Fig. 2: Oxidative decarboxylation.
Explanation:
The oxidative decarboxylation is occurring that converts Ketoisocaproate to Isovaleryl-CoA. The cofactors involved are “NAD+ (Nicotinamide adenine dinucleotide)”, “TPP (Triphenyl phosphate)” and “FAD (Flavin adenine dinucleotide)”, and “lipoate”. The formation of acetyl CoA by oxidative decarboxylation of pyruvate is similar to this given reaction.
(c)
To determine: The type of reaction in which Isovaleryl-CoA is converted to β-methylcrotonyl-CoA.
Introduction:
The series of metabolic reactions, in which the energy stored in the compounds is released for the production of ATP (adenosine triphosphate), is called “Krebs’s cycle”. The pyruvate undergoes series of reactions in the citric acid cycle and converts into Acetyl CoA (acetyl coenzyme A) in the presence of oxygen.
β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(c)
Explanation of Solution
Pictorial representation: Fig. 3 represents the dehydrogenation reaction.
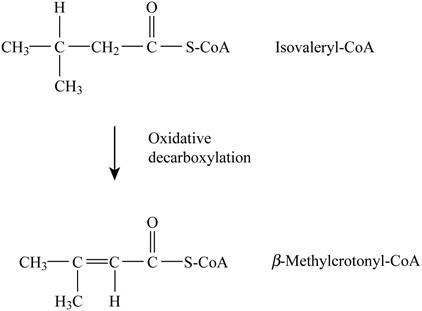
Fig. 3: Dehydrogenation reaction.
Explanation:
The type of reaction in which Isovaleryl-CoA is converted to β-methylcrotonyl-CoA is dehydrogenation. The cofactor is (FAD) Flavin adenine
(d)
To determine: The type of reaction in which beta methylcrotonyl-CoA is converted to beta-methylglutaconyl-CoA.
Introduction:
The series of metabolic reactions, in which the energy stored in the compounds is released for the production of ATP (adenosine triphosphate), is called “Krebs’s cycle”. The pyruvate undergoes series of reactions in the citric acid cycle and converts into Acetyl CoA (acetyl coenzyme A) in the presence of oxygen. β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(d)
Explanation of Solution
Pictorial representation: Fig. 4 represents the carboxylation reaction.
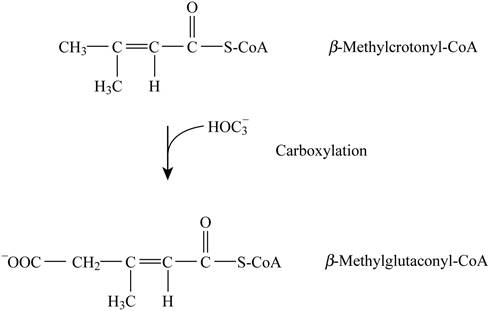
Fig. 4: Carboxylation reaction.
Explanation:
The formation of β-methylglutaconyl-CoA by β-methylcrotonyl-CoA is a type of carboxylation reaction. The cofactors are biotin and ATP. This step is not analogous to any step in β-oxidation and citric acid cycle.
(e)
To determine: The type of reaction in which beta-methylglutaconyl-CoA is converted to β-hydroxy β-methylglutaryl-CoA.
Introduction:
The series of metabolic reactions, in which the energy stored in the compounds is released for the production of ATP (adenosine triphosphate), is called “Krebs’s cycle”. The pyruvate undergoes series of reactions in the citric acid cycle and converts into Acetyl CoA (acetyl coenzyme A) in the presence of oxygen. β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(e)
Explanation of Solution
Pictorial representation: Fig. 5 represents the hydration reaction.
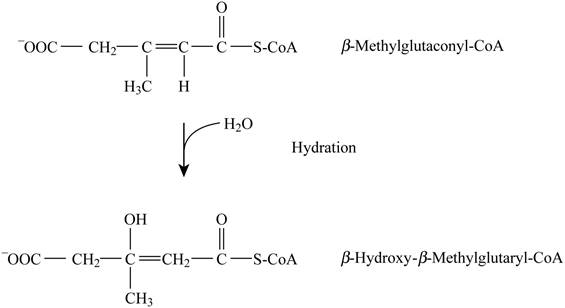
Fig. 5: Hydration reaction.
Explanation:
The conversion of beta-methylglutaconyl-CoA into β-hydroxy β-methylglutaryl-CoA is a type of hydration reaction. This reaction does not require the cofactors. The formation of malate by fumarate and formation of 3-hydroxyacyl-CoA by enoyl-CoA are analogous to this given reaction.
(f)
To determine: The type of reaction in which β-hydroxy β-methylglutaryl-CoA is converted to acetyl-CoA and acetoacetate.
Introduction:
The series of metabolic reactions, in which the energy stored in the compounds is released for the production of ATP (adenosine triphosphate), is called “Krebs’s cycle”. The pyruvate undergoes series of reactions in the citric acid cycle and converts into Acetyl CoA (acetyl coenzyme A) in the presence of oxygen.
β-oxidation is the oxidation of fatty acids in cells in absence of glucose and glycogen, to produce ATP. Oxidation takes place at the β carbon, thus, this reaction is termed as β-oxidation.
(f)
Explanation of Solution
Pictorial representation: Fig. 6 represents the reverse aldol reaction.
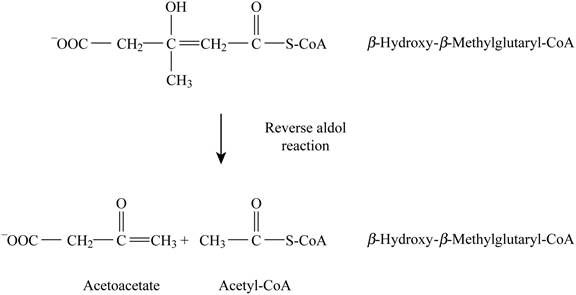
Fig. 6: Reverse aldol reaction.
Explanation:
The formation of acetate-CoA and acetoacetate by β-hydroxy β-methylglutaryl-CoA is a type of reverse aldol reaction. There are no cofactors involved in this reaction. The analogous reaction is the opposite reaction involving citrate synthase. This reaction is analogous to citrate synthase reaction in the citric acid cycle. It is identical to the cleavage of β-hydroxy-β-methylglutaryl-CoA in the formation of
Want to see more full solutions like this?
Chapter 18 Solutions
EBK LEHNINGER PRINCIPLES OF BIOCHEMISTR
- The following data were recorded for the enzyme catalyzed conversion of S -> P. Question: Estimate the Vmax and Km. What would be the rate at 2.5 and 5.0 x 10-5 M [S] ?arrow_forwardPlease helparrow_forwardThe following data were recorded for the enzyme catalyzed conversion of S -> P Question: what would the rate be at 5.0 x 10-5 M [S] and the enzyme concentration was doubled? Also, the rate given in the table is from product accumulation after 10 minuets of reaction time. Verify these rates represent a true initial rate (less than 5% turnover). Please helparrow_forward
- The following data was obtained on isocitrate lyase from an algal species. Identify the reaction catalyzed by this enzyme, deduce the KM and Vmax , and determine the nature of the inhibition by oxaloacetate. Please helparrow_forwardIn the table below, there are sketches of four crystals made of positively-charged cations and negatively-charged anions. Rank these crystals in decreasing order of stability (or equivalently increasing order of energy). That is, select "1" below the most stable (lowest energy) crystal. Select "2" below the next most stable (next lowest energy) crystal, and so forth. A B 鹽 (Choose one) +2 C +2 +2 (Choose one) D 鹽雞 (Choose one) (Choose one)arrow_forward1. Draw the structures for the fats A. 16:2: w-3 and B. 18:3:49,12,15 2. Name each of the molecules below (image attached)arrow_forward
- draw the structures for the fats A. 16:2:w-3 B 18:3:9,12,15arrow_forward1. Below is a template strand of DNA. Show the mRNA and protein that would result. label the ends of the molecules ( refer to attached image)arrow_forwardAttach the followina labels to the diagram below: helicase, single stranded binding proteins, lagging strand, leading strand, DNA polymerase, primase, 5' ends (3), 3' ends (3) (image attached)arrow_forward
- 1. How much energy in terms of ATP can be obtained from tristearin (stearate is 18:0) Show steps pleasearrow_forwardMultiple choice urgent!!arrow_forward1. Write the transamination reaction for alanine. Indicate what happens next to each of the molecules in the reaction, and under what conditions it happens. 2.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





