
(a)
Interpretation:
The synthesis of
Concept introduction:
Nitration is a process in which an
Answer to Problem 18.69AP
The reaction of benzene and chlorine gas in the presence of iron chloride followed by nitration forms
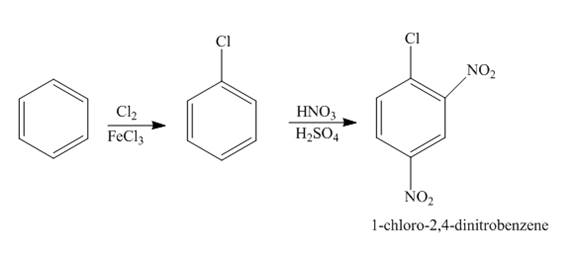
Explanation of Solution
The chlorination of benzene using chlorine gas in the presence of iron chloride forms chlorobenzene which further reacts with nitric acid in the presence of sulphuric acid to give
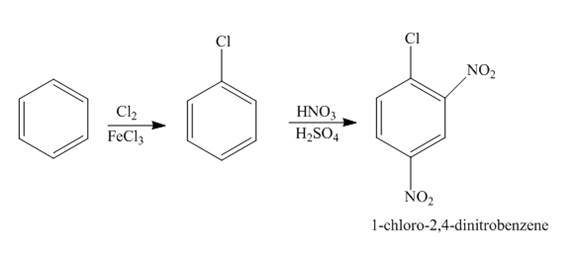
Figure 1
The reaction of benzene and chlorine gas in the presence of iron chloride followed by nitration forms
(b)
Interpretation:
The synthesis of
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution in the presence of concentrated sulfuric acid and concentrated nitric acid. Electron donating groups substituted on the aromatic ring are those which donate electron to the aromatic ring. Electron donating groups are ortho and para-directing.
Answer to Problem 18.69AP
The reaction of benzene and nitric acid in the presence of sulphuric acid followed by the treatment with
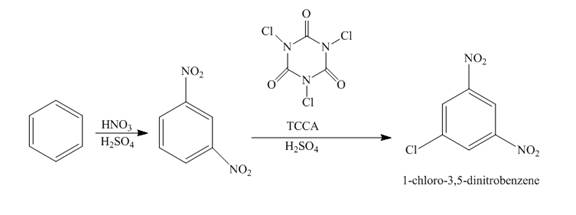
Explanation of Solution
The reaction of benzene and nitric acid in the presence of sulphuric acid forms

Figure 2
The reaction of benzene and nitric acid in the presence of sulphuric acid followed by the treatment with
(c)
Interpretation:
The synthesis of diphenylacetylene from
Concept introduction:
The addition of a bromine atom in a compound is known as bromination. Bromination occurs through electrophilic substitution reaction. Bromine atom acts as an electrophile which causes the formation of sigma bond in the reaction.
Answer to Problem 18.69AP
The reaction of
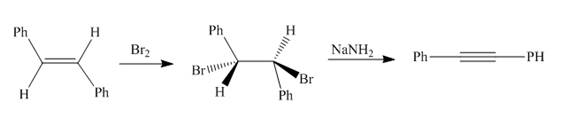
Explanation of Solution
The reaction of

Figure 3
The reaction of
(d)
Interpretation:
The synthesis of
Concept introduction:
The replacement or substitution of one
Answer to Problem 18.69AP
The reaction of chlorobenzene and acetyl chloride in the presence of
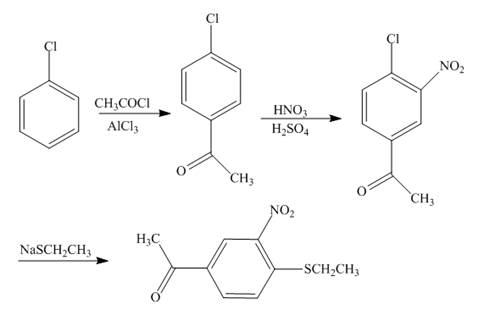
Explanation of Solution
The reaction of chlorobenzene and acetyl chloride in the presence of
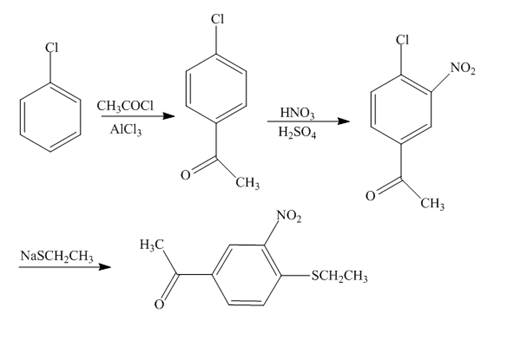
Figure 4
The reaction of chlorobenzene and acetyl chloride in the presence of
(e)
Interpretation:
The synthesis of
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution in the presence of concentrated sulfuric acid and concentrated nitric acid. Electron donating groups substituted on the aromatic ring are those which donate electron to the aromatic ring. Electron donating groups are ortho and para-directing.
Answer to Problem 18.69AP
The reaction of chlorobenzene undergoes nitration followed by the addition of a base which further oxidizes with the help of
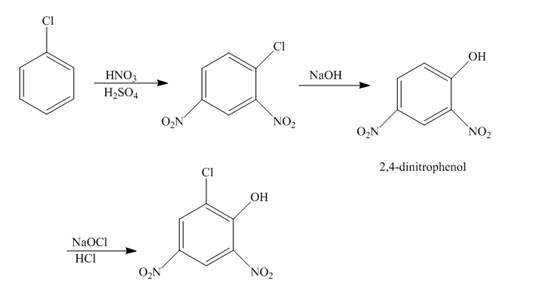
Explanation of Solution
The reaction of chlorobenzene and nitric acid in the presence of sulphuric acid undergoes nitration forms
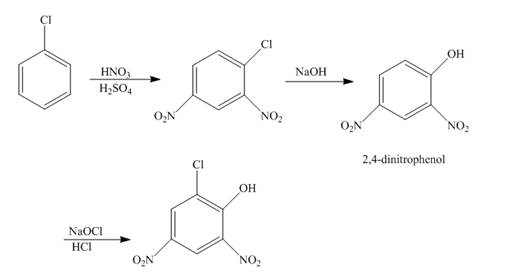
Figure 5
The reaction of chlorobenzene undergoes nitration followed by the addition of a base which further oxidizes with the help of
(f)
Interpretation:
The synthesis of butylated hydroxytoluene (BHT) from
Concept introduction:
A chemical reaction that involves the removal of a water molecule from the reacting molecule is known as a dehydration reaction. It takes place in the presence of a strong acid. The percentage of product formed is determined by Saytzeff’s rule.
Answer to Problem 18.69AP
The reaction of
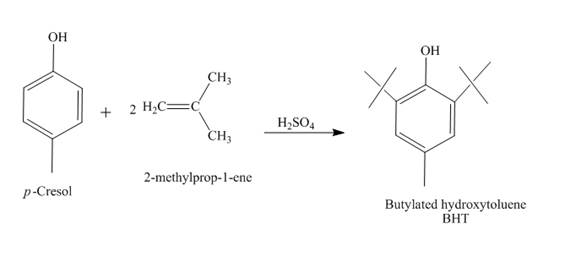
Explanation of Solution
The reaction between

Figure 6
The reaction of
(g)
Interpretation:
The synthesis of the given product from catechol and other reagents is to be predicted.
Concept introduction:
A chemical reaction that involves the removal of a water molecule from the reacting molecule is known as a dehydration reaction. It takes place in the presence of a strong acid. The percentage of product formed is determined by Saytzeff’s rule.
Answer to Problem 18.69AP
The reaction of catechol and
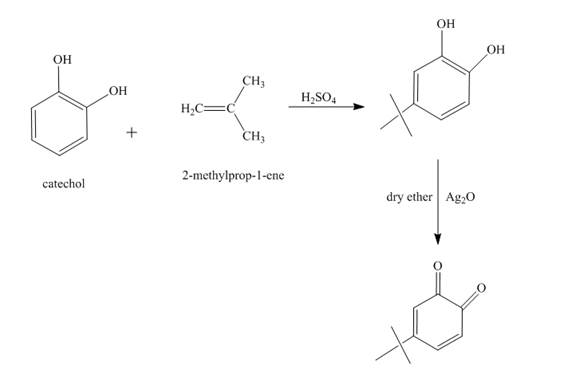
Explanation of Solution
The reaction of catechol and

Figure 7
The reaction of catechol and
(h)
Interpretation:
The synthesis of
Concept introduction:
Grignard reagents are
Answer to Problem 18.69AP
The reaction of bromobenzene with Grignard reagent and ethylene oxide forms
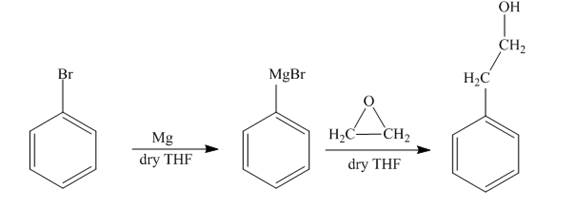
Explanation of Solution
The reaction of bromobenzene with magnesium in the presence of dry
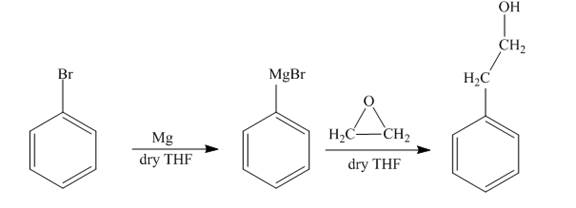
Figure 8
The synthesis of
(i)
Interpretation:
The synthesis of given product from
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution in the presence of concentrated sulfuric acid and concentrated nitric acid. Electron donating groups substituted on the aromatic ring are those which donate electron to the aromatic ring. Electron donating groups are ortho and para-directing.
Answer to Problem 18.69AP
The reaction of fluorobenzene undergoes nitration followed by the addition of
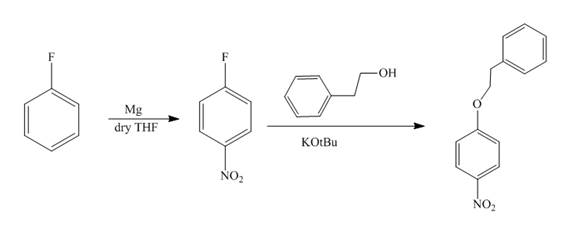
Explanation of Solution
The reaction of fluorobenzene with nitric acid in the presence of sulphuric acid forms

Figure 9
The reaction of fluorobenzene undergoes nitration followed by the addition of
(j)
Interpretation:
The synthesis of the given product from benzene, ethylene group and other reagents is to be predicted.
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution in the presence of concentrated sulfuric acid and concentrated nitric acid. Electron donating groups substituted on the aromatic ring are those which donate electron to the aromatic ring. Electron donating groups are ortho and para-directing.
Answer to Problem 18.69AP
The synthesis of the desired product from benzene and ethylene group is shown below.
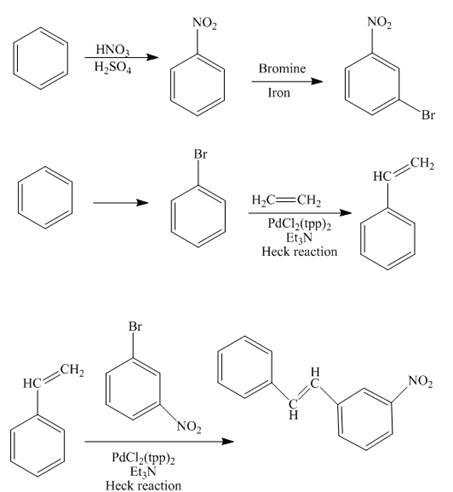
Explanation of Solution
The reaction of benzene with nitric acid in the presence of sulphuric acid forms nitrobenzene which further reacts with bromine to form
The styrene is prepared by the bromination of benzene followed by the addition of ethane in the presence of
In the final step, the compound
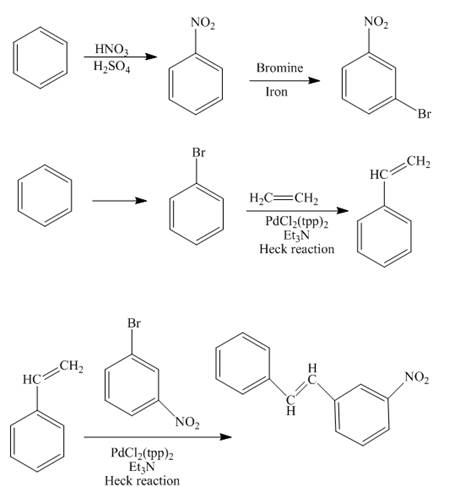
Figure 10
The synthesis of the desired product from benzene and ethylene group as shown in Figure 10.
(k)
Interpretation:
The synthesis of the given product from
Concept introduction:
The treatment of unsaturated halide with an alkene in the presence of a base and a
Answer to Problem 18.69AP
The reaction of
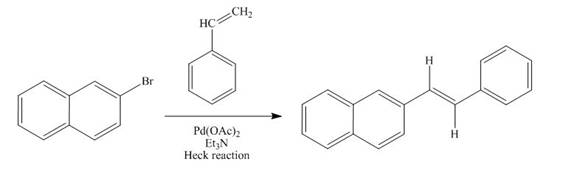
Explanation of Solution
The

Figure 11
The reaction of
(l)
Interpretation:
The synthesis of the given product from bromobenzene, ethylene group and other reagents is to be predicted.
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution in the presence of concentrated sulfuric acid and concentrated nitric acid.
Answer to Problem 18.69AP
The reaction of bromobenzene with nitric acid in the presence of sulphuric acid followed by coupling reaction with allyl alcohol to form the desired product is shown below.
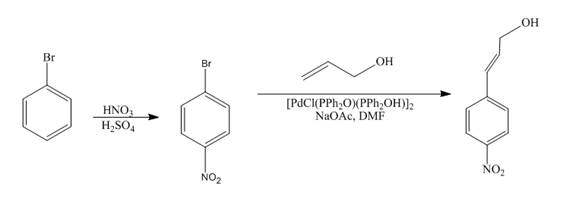
Explanation of Solution
The reaction of bromobenzene with nitric acid in the presence of sulphuric acid forms

Figure 12
The reaction of bromobenzene with nitric acid in the presence of sulphuric acid followed by coupling reaction with allyl alcohol to form the desired product as shown in Figure 12.
(m)
Interpretation:
The synthesis of the given product from
Concept introduction:
The hydroxide ions acts as a poor leaving groups as well as strong bases. The tosyl chloride reacts with hydroxide ions in the presence of weak base like pyridine to convert in a good leaving groups.
Answer to Problem 18.69AP
The reaction of
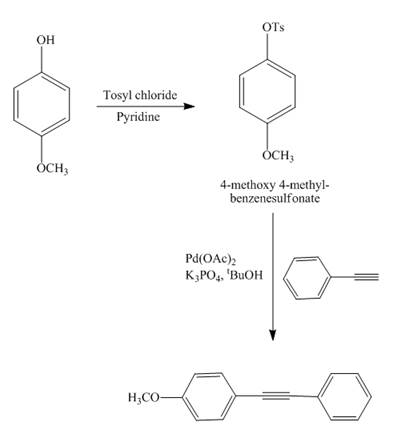
Explanation of Solution
The reaction of
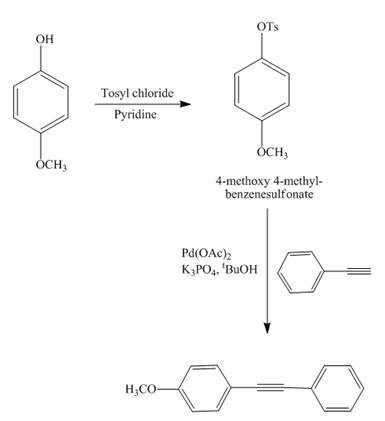
Figure 13
The reaction of
(n)
Interpretation:
The synthesis of the given product from
Concept introduction:
Sonogashira coupling is used to form carbon-carbon bond in the chemical reaction.
The addition of bromine atom in a compound is known as bromination. Bromination is occurs through elctrophilic substitution reaction. Bromine atom acts as a electrophile which causes the formation of sigma bond in the reaction.
Answer to Problem 18.69AP
The synthesis of the desired product from
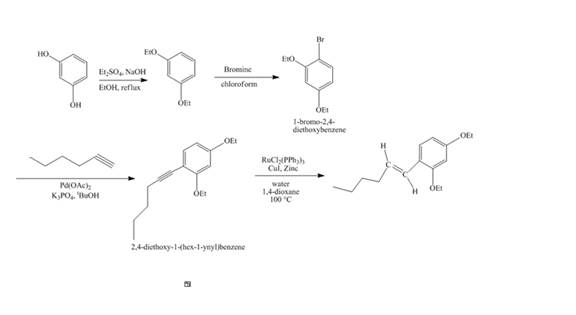
Explanation of Solution
The reaction of recorcinol with

Figure 14
The synthesis of the desired product from
(o)
Interpretation:
The synthesis of the given product from iodobenzene and other reagents is to be predicted.
Concept introduction:
The coupling reactions used to connect two compounds with the help of a metal catalyst like palladium. Sonogashira coupling is used to form a carbon-carbon bond in the chemical reaction.
Answer to Problem 18.69AP
The synthesis of the desired product from iodobenzene and cyclohexene is shown below.
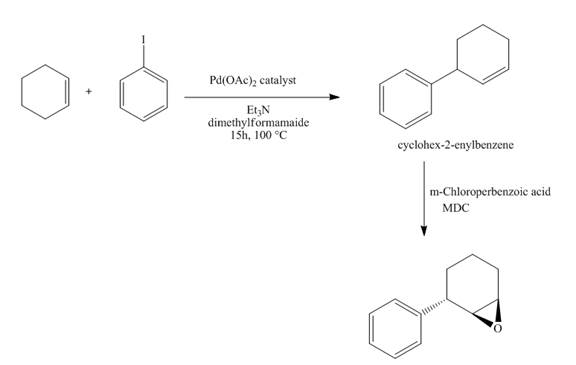
Explanation of Solution
The reaction of iodobenzene with cyclohexene in the presence of
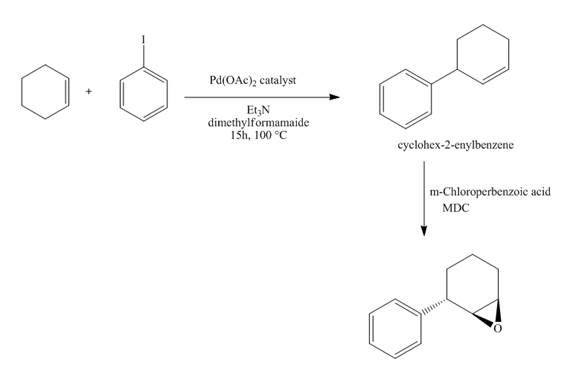
Figure 15
The synthesis of the desired epoxide compound from iodobenzene and cyclohexene is shown below in Figure 15.
Want to see more full solutions like this?
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY
- An expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.arrow_forwardThe reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?arrow_forwardOne liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forward
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





