
University Physics (14th Edition)
14th Edition
ISBN: 9780133969290
Author: Hugh D. Young, Roger A. Freedman
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.59P
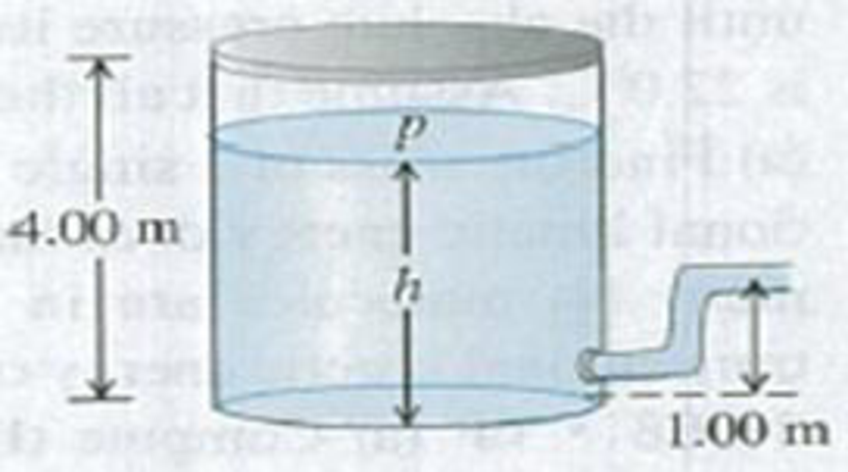
CP A large tank of water has a hose connected to it (Fig. P18.59). The tank is sealed at the top and has compressed air between the water surface and the top. When the water height h has the value 3.50 m, the absolute pressure p of the compressed air is 4.20 × 105 Pa. Assume that I he air above the water expands at constant temperature, and take the atmospheric pressure to be 1.00 × 105 Pa. (a) What is the speed with which water flows out of the hose when h = 3.50 m? (b) As water flows out of the tank, h decreases. Calculate the speed of flow for h = 3.00 m and for h = 2.00 m. (c) At what value of h does the flow stop?
Figure P18.59
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Just 5 and 6 don't mind 7
In an electron gun, electrons are accelerated through a region with an electric field of magnitude 1.5 × 104 N/C for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?
Please solve and answer this problem correctly please. Thank you!!
Chapter 18 Solutions
University Physics (14th Edition)
Ch. 18 - Section 18.1 states that ordinarily, pressure,...Ch. 18 - In the ideal-gas equation, could an equivalent...Ch. 18 - When a car is driven some distance, the air...Ch. 18 - The coolant in an automobile radiator is kept at a...Ch. 18 - Unwrapped food placed in a freezer experiences...Ch. 18 - A group of students drove from their university...Ch. 18 - The derivation of the ideal-gas equation included...Ch. 18 - A rigid, perfectly insulated container has a...Ch. 18 - (a) Which has more atoms: a kilogram of hydrogen...Ch. 18 - Use the concepts of the kinetic-molecular model to...
Ch. 18 - The proportions of various gases in the earths...Ch. 18 - Comment on the following statement: When two gases...Ch. 18 - Prob. Q18.13DQCh. 18 - The temperature of an ideal gas is directly...Ch. 18 - If the pressure of an ideal monatomic gas is...Ch. 18 - In deriving the ideal-gas equation from the...Ch. 18 - Imagine a special air filter placed in a window of...Ch. 18 - A gas storage tank has a small leak. The pressure...Ch. 18 - Consider two specimens of ideal gas at the same...Ch. 18 - The temperature of an ideal monatomic gas is...Ch. 18 - Prob. Q18.21DQCh. 18 - (a) If you apply the same amount of heat to 1.00...Ch. 18 - Prob. Q18.23DQCh. 18 - In a gas that contains N molecules, is it accurate...Ch. 18 - The atmosphere of the planet Mars is 95.3% carbon...Ch. 18 - Prob. Q18.26DQCh. 18 - Ice is slippery to walk on, and especially...Ch. 18 - Hydrothermal vents are openings in the ocean floor...Ch. 18 - The dark areas on the moons surface are called...Ch. 18 - In addition to the normal cooking directions...Ch. 18 - A 20.0-L tank contains 4.86 104 kg of helium at...Ch. 18 - Helium gas with a volume of 3.20 L, under a...Ch. 18 - A cylindrical tank has a tight-fitting piston that...Ch. 18 - A 3.00-L lank contains air at 3.00 atm and 20.0C....Ch. 18 - Planetary Atmospheres. (a) Calculate the density...Ch. 18 - You have several identical balloons. You...Ch. 18 - A Jaguar XK8 convertible has an eight-cylinder...Ch. 18 - A welder using a tank of volume 0.0750 m3 fills it...Ch. 18 - A large cylindrical tank contains 0.750 m3 of...Ch. 18 - An empty cylindrical canister 1.50 m long and 90.0...Ch. 18 - The gas inside a balloon will always have a...Ch. 18 - An ideal gas has a density of 1.33 106 g/cm3 at...Ch. 18 - If a certain amount of ideal gas occupies a volume...Ch. 18 - A diver observes a bubble of air rising from the...Ch. 18 - A metal tank with volume 3.10 L will burst if the...Ch. 18 - Three moles of an ideal gas are in a rigid cubical...Ch. 18 - With the assumptions of Example 18.4 (Section...Ch. 18 - With the assumption that the air temperature is a...Ch. 18 - (a) Calculate the mass of nitrogen present in a...Ch. 18 - At an altitude of 11,000 m (a typical cruising...Ch. 18 - Prob. 18.21ECh. 18 - Prob. 18.22ECh. 18 - Modern vacuum pumps make it easy to attain...Ch. 18 - The Lagoon Nebula (Fig. E18.24) is a cloud of...Ch. 18 - In a gas at standard conditions, what is the...Ch. 18 - How Close Together Are Gas Molecules? Consider an...Ch. 18 - (a) What is the total translational kinetic energy...Ch. 18 - A flask contains a mixture of neon (Ne), krypton...Ch. 18 - We have two equal-size boxes, A and B. Each box...Ch. 18 - A container with volume 1.64 L is initially...Ch. 18 - Prob. 18.31ECh. 18 - Martian Climate. The atmosphere of Mars is mostly...Ch. 18 - Prob. 18.33ECh. 18 - Calculate the mean free path of air molecules at...Ch. 18 - At what temperature is the root-mean-square speed...Ch. 18 - Prob. 18.36ECh. 18 - Prob. 18.37ECh. 18 - Perfectly rigid containers each hold n moles of...Ch. 18 - (a) Compute the specific heat at constant volume...Ch. 18 - Prob. 18.40ECh. 18 - Prob. 18.41ECh. 18 - For a gas of nitrogen molecules (N2), what must...Ch. 18 - Prob. 18.43ECh. 18 - Meteorology. The vapor pressure is the pressure of...Ch. 18 - Calculate the volume of 1.00 mol of liquid water...Ch. 18 - A physics lecture room at 1.00 atm and 27.0C has a...Ch. 18 - CP BIO The Effect of Altitude on the Lungs. (a)...Ch. 18 - CP BIO The Bends. If deep-sea divers rise to the...Ch. 18 - CP A hot-air balloon stays aloft because hot air...Ch. 18 - In an evacuated enclosure, a vertical cylindrical...Ch. 18 - A cylinder 1.00 m tall with inside diameter 0.120...Ch. 18 - CP During a test dive in 1939, prior to being...Ch. 18 - Atmosphere or Titan. Titan, the largest satellite...Ch. 18 - Pressure on Venus. At the surface of Venus the...Ch. 18 - An automobile tire has a volume of 0.0150 m3 on a...Ch. 18 - A flask with a volume of 1.50 L, provided with a...Ch. 18 - CP A balloon of volume 750 m3 is to be filled with...Ch. 18 - A vertical cylindrical tank contains 1.80 mol of...Ch. 18 - CP A large tank of water has a hose connected to...Ch. 18 - CP A light, plastic sphere with mass m = 9.00 g...Ch. 18 - Prob. 18.61PCh. 18 - BIO A person at rest inhales 0.50 L of air with...Ch. 18 - You have two identical containers, one containing...Ch. 18 - The size of an oxygen molecule is about 2.0 1010...Ch. 18 - A sealed box contains a monatomic ideal gas. The...Ch. 18 - Helium gas is in a cylinder that has rigid walls....Ch. 18 - You blow up a spherical balloon to a diameter of...Ch. 18 - CP (a) Compute the increase in gravitational...Ch. 18 - Prob. 18.69PCh. 18 - Prob. 18.70PCh. 18 - It is possible to make crystalline solids that are...Ch. 18 - Hydrogen on the Sun. The surface of the sun has a...Ch. 18 - Prob. 18.73PCh. 18 - Planetary Atmospheres. (a) The temperature near...Ch. 18 - Prob. 18.75PCh. 18 - Prob. 18.76PCh. 18 - CALC (a) Explain why in a gas of N molecules, the...Ch. 18 - Prob. 18.78PCh. 18 - CP Oscillations of a Piston. A vertical cylinder...Ch. 18 - DATA A steel cylinder with rigid walls is evacuate...Ch. 18 - DATA The Dew Point and Clouds. The vapor pressure...Ch. 18 - DATA The statistical quantities average value and...Ch. 18 - CP Dark Nebulae and the Interstellar Medium. The...Ch. 18 - CALC Earths Atmosphere. In t he troposphere, the...Ch. 18 - Prob. 18.85PPCh. 18 - Estimate the ratio of the thermal conductivity of...Ch. 18 - The rate of effusionthat is, leakage of a gas...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Please solve and answer this problem correctly please. Thank you!!arrow_forwarda) Use the node-voltage method to find v1, v2, and v3 in the circuit in Fig. P4.14. b) How much power does the 40 V voltage source deliver to the circuit? Figure P4.14 302 202 w w + + + 40 V V1 80 Ω 02 ΣΑΩ 28 A V3 + w w 102 202arrow_forwardPlease solve and answer this problem correctly please. Thank you!!arrow_forward
- You're on an interplanetary mission, in an orbit around the Sun. Suppose you make a maneuver that brings your perihelion in closer to the Sun but leaves your aphelion unchanged. Then you must have Question 2 options: sped up at perihelion sped up at aphelion slowed down at perihelion slowed down at aphelionarrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE DO NOT USE LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forward
- No chatgpt pls will upvotearrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardNo chatgpt pls will upvotearrow_forward
- No chatgpt pls will upvotearrow_forwardSolve and answer the question correctly please. Thank you!!arrow_forward་ The position of a particle is described by r = (300e 0.5t) mm and 0 = (0.3t²) rad, where t is in seconds. Part A Determine the magnitude of the particle's velocity at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. v = Value Submit Request Answer Part B ? Units Determine the magnitude of the particle's acceleration at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. a = Value A ? Unitsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY