
Concept explainers
How many moles of electrons are required to produce (a) 0.84 L of O2 at exactly 1 atm and 25°C from aqueous H2SO4 solution; (b) 1.50 L of Cl2 at 750 mmHg and 20°C from molten NaCl; (c) 6.0 g of Sn from molten SnCl2?
(a)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 0.84L of O2 with 1 atm pressure upon electrolysis of aqueous H2SO4 at 25oC.
Concept introduction:
Electrolysis of aqueous sulfuric acid i.e. acidified water resulted in the production of oxygen and hydrogen gas, which will be liberated at the anode and cathode respectively. The presence of H+ and SO-4 made the solution to be more conductivity. SO-4 is more stable to be inert at the anode.
Further the cell reaction can be written as shown below
Since volume and pressure of oxygen produced was given, by applying it into ideal gas equation we can calculate the number of mole of oxygen produced
The ideal gas equation can be given as follows.
On applying the number of moles of oxygen produced into stoichiometry of the reaction, the number of moles electron involved in the reaction can be calculated. In case of the given reaction 4 mole of electron was liberated during the production of one mole of oxygen. Since one Faraday is equal to one mole of electron, so 4 Faraday of electricity will be needed to produce one mole of oxygen.
Finally the Faraday of electricity utilized to produce the required amount of oxygen can be calculated according the formula
To find: Amount of Faraday of electricity need to produce 0.076L of O2 with pressure 755mmHg, at 298K, through electrolysis of water.
Answer to Problem 18.52QP
Ideal gas equation can be used to calculate the number of moles of oxygen produced, from that faraday of electricity needed will be calculated in successive steps (a)
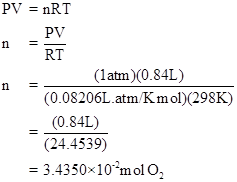
Since one mole of oxygen need 4 Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 0.84L of oxygen with pressure 1 atm was calculated as
Explanation of Solution
Ideal gas equation can be used to calculate the number of moles of oxygen produced
Since one mole of oxygen need 4 Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 0.84L of oxygen with pressure 1 atm was calculated as
At first the number of moles of oxygen produced through electrolysis was calculated using ideal gas equation, from the given volume and pressure. Thus it was calculated as
The amount of electricity needed to produce 0.84L of oxygen with pressure of 1atm was determined to be
(b)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 1.50L of Cl2 with pressure 750 mmHg at 20oC by electrolysis of molten NaCl.
Concept introduction:
Electrolysis of molten sodium chloride was represented by the below equation. By using ideal gas equation, the number of moles of chlorine liberated at the anode can be calculated, provided the pressure and volume are known.
The ideal gas equation can be given as follows.
The equation we find that two mole of electron is needed to produce one mole of chlorine gas , Since one Faraday is equal to one mole of electron, The Faraday of electricity utilized to produce given amount of chlorine can be calculated according the formula
To find: Faraday of electricity need to produce 1.50 L of Cl2 with pressure 750mmHg, at 293K, through electrolysis of molten NaCl.
Answer to Problem 18.52QP
Ideal gas equation can be used to calculate the number of moles of chlorine gas produced, Further from the number of moles of chlorine; the faraday of electricity utilized will be calculated in successive steps (b)
Since one mole of chlorine need two Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 1.50L of chlorine with pressure 750mm Hg was calculated as
Explanation of Solution
Ideal gas equation can be used to calculate the number of moles of oxygen produced
Since one mole of chlorine need two Faraday of electricity, so the Faraday of electricity needed to produce
The number of moles of chlorine produced through electrolysis was calculated using ideal gas equation, from the given volume and pressure it was calculated as
The amount of electricity needed to produce 1.5 L of chlorine with pressure of 750 mmHg was determined to be
(c)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 6g of Sn though electrolysis of molten SnCl2.
Concept introduction:
Electrolysis of molten stannous chloride will result in the formation of Tin (Sn) and chlorine gas and half-cell reaction at the anode and cathode was given below. In this two electrons were released by chloride ion at the anode and get liberated as chlorine gas, further stannous ion accept two electron and for tin metal
The ideal gas equation can be given as follows.
Since the mass of the metal produced was given, from that number of moles of Sn can be calculated. Further from the number of moles of Sn produced the Faraday of electricity can be calculated by the formula given below, since one Faraday is equal to one mole of electron. In the present case 2 moles of electrons are needed to reduce one mole of Sn2+
So
To find: Faraday of electricity need to produce 6 g of Tin, by electrolysis of molten SnCl2.
Answer to Problem 18.52QP
From the mass of tin produced during the electrolysis, the Faraday of electricity needed for reaction can be calculated in the following steps (c).
Since one mole of Sn2+ ion need two Faraday of electricity to, so the Faraday of electricity needed to produce
Explanation of Solution
From the molar mass calculation
Weight = 6g
Atomic Mass = 118.7g
Since one mole of Sn2+ ion need two Faraday of electricity, so the Faraday of electricity needed to produce
The number of moles of tin produced through electrolysis was calculated as
The Faraday of electricity need to produce 6g of Tin from by electrolysis of molten SnCl2 was identified as
Want to see more full solutions like this?
Chapter 18 Solutions
Loose Leaf for Chemistry
- How would you use infrared spectroscopy to distinguish between the following pairs of constitutional isomers? (a) CH3C=CCH3 || and CH3CH2C=CH (b) CH3CCH=CHCH3 and CH3CCH2CH=CH2 Problem 12-41 The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can. (a) 100 Relative abundance (%) 80 60 60 40 200 20 (b) 100 Transmittance (%) 10 20 20 80- 60- 40- 20 40 60 80 100 120 140 m/z 500 4000 3500 3000 2500 2000 1500 Wavenumber (cm-1) 1000arrow_forwardPropagation of uncertainty. You have a stock solution certified by the manufacturer to contain 150.0±0.03 µg SO42-/mL. You would like to dilute it by a factor of 100 to obtain 1.500 µg/mL. Calculate the uncertainty in the two methods of dilution below. Use the following uncertainty values for glassware: Glassware Uncertainty (assume glassware has been calibrated and treat the values below as random error) 1.00 mL volumetric pipet 0.01 mL 10.00 mL volumetric pipet 0.02 mL 100.00 mL volumetric flask 0.08 mL Transfer 10.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask. Then take 10.00 mL of the resulting solution and dilute it a second time with a 100 mL flask. 2. Transfer 1.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask.arrow_forwardDraw all resonance structures for the following ion: CH₂ Draw all resonance structures on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default. 2D ד CONT HD EXP CON ? 1 [1] Α 12 Marvin JS by Chemaxon A DOO H C N Br I UZ OSPFarrow_forward
- What is the average mass of the 10 pennies? Report your value with correct significant figures. What is the error (uncertainty) associated with each mass measurement due to the equipment? What is the uncertainty associated with the average value? Note that the uncertainty of the balance will propagate throughout the calculation. What is the standard deviation of the 10 mass measurements? Explain the difference between the propagated uncertainty and the standard deviation. Which number would you use to describe the uncertainty in the measurement? Calculate the total mass of the pennies with associated uncertainty. Calculate the average density of a penny based on these data. Propagate the uncertainty values for both mass and volume in your calculations.arrow_forwardCan you help me and explain the answers please.arrow_forwardB 1 of 2 Additional problems in preparation to Midterm #1: 1.) How can the following compounds be prepared using Diels-Alder reaction: CH3 O CN (a) (b) CN CH3 2.) What is the missing reagent in the shown reaction? H3C + ? H3C H3C CN H3C ''CN (၁) H 3.) Write the products 1,2-addition and 1,4-addition of DBr to 1,3-cyclohexadiene. Remember, D is deuterium, a heavy isotope of hydrogen. It reacts exactly like hydrogen. 4.) In the shown reaction, which will be the kinetic product and which will be the thermodynamic product? H3C CI H3C HCI H3C + 5.) Which of the following molecules is aromatic? (a) (b) (c) H 6.) Which of the following molecules is aromatic? (a) (b) (c) 7.) Write the mechanism for the shown reaction. + Ха AICI 3 CI 8.) Suggest reagents that would convert benzene into the shown compounds. CI NO2 -8-6-6-8-a (a) (b) (c) (d) (e) (a) SO3H Brarrow_forward
- The number of 2sp^2 hybridized atoms in is: A. 8; B. 6; C.4; D.2; E.0;arrow_forwardThe highest boiling compound from among the following isA. 2-methylheptane; B. 3-methylheptane; C. 2,2-dimethylhexane;D. octane; E. 2,2,3-trimethylpentanearrow_forwardWhich of the following features are found in the most stable structure ofCH5NO that does not have a CO bond?w. a π bond, x. two NH bonds, y. one OH bond, z. 3 lone pairsA. w, x; B. x, y; C. y, z; D. x, y, z; E. all of them.arrow_forward
- Which one of the following functional groups is not present in thecompound shownA. amine; B. aldehyde, C. ether; D. amide. E. ketonearrow_forwardWhich of the following formulas correspond to at least one compound inwhich resonance is important?w. C2H5N x. C3H5Br; y. C3H4; z. C4H6.A. w, x, y; B. x, y, z; C. w, x, z; D. w, y, z; E. all of themarrow_forwardPredict the product(s) that are formed after each step for reactions 1-4. In each case, consider formation of any chiral center(s) and draw all expected stereoisomers. 1) OH 1) HBr (SN2) 2) NaOH, heat 3) BH3, THF 4) H2O2, NaOH 2) OH 1) SOCI 2, py 2) NaOEt 3) Br2, H₂O 3) OH 1) H2SO4 conc. 2) HBr, ROOR 3) KOtBu 4) OH 1) TsCl, py 2) NaOEt 3) 03 4) DMSarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





