
Concept explainers
The zinc-air battery shows much promise for electric cars because it is lightweight and rechargeable:
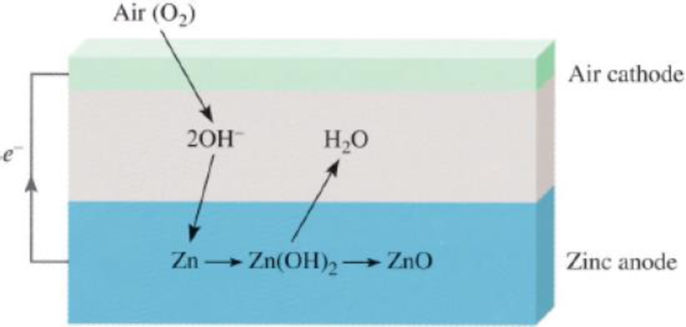
The net transformation is
(a)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The half cell reactions of the given cell,
The standard
Explanation of Solution
To record the given data
To write the half cell reactions and overall reaction
The half cell reactions are,
Overall reaction,
To find the
The of
To find the
Using the value of free energy and the number of electrons transferred the
On rearranging the equation we get,
(b)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The
Explanation of Solution
To record the given data
Partial pressure of oxygen
To calculate the
The
(c)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The energy density of the zinc electrode is found to be
Explanation of Solution
To record the given data
Amount of zinc
Molecular weight of zinc
To calculate the number of moles of zinc
Number of moles of zinc in
To calculate the energy density of zinc electrode
Free energy is the maximum amount of energy in the system that can be converted into useful work. Energy density can be obtained by multiplying the free energy value with the number of moles of zinc.
(d)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The amount of air supplied to the battery in each second is found to be
Explanation of Solution
To record the given data
Amount of current derived from the cell
To calculate the number of moles of electrons required for producing given amount of charge
Charge produced and the numbers of moles of electrons transferred are related by the following equation.
The number of moles of electrons transferred,
To calculate the number of moles of oxygen gas reduced by
From the equation for the cell reaction we have seen that
To calculate the volume of oxygen when the partial pressure is
The volume of oxygen at
To calculate the volume of air required at each second.
The volume of air required at each second is found as given below.
Want to see more full solutions like this?
Chapter 18 Solutions
Loose Leaf for Chemistry
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





