
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
12th Edition
ISBN: 9781337915977
Author: Bettelheim
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18, Problem 12P
Interpretation Introduction
Interpretation:
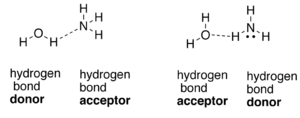
To draw short sections of two parallel chains of nylon-66(each running in the same direction) and if it is possible to align them in such a way that there is hydrogen bonding between the N-H groups of one chain and the C=O groups of the parallel chain should be explained.
Concept Introduction:
A hydrogen bond is formed by a partial electrostatic attraction between hydrogen (H) atom attached to an electronegative atom (nitrogen (N), oxygen (O), or fluorine (F)) and other electronegative atom and lone pair present in the vicinity of it.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me solve this reaction.
Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.
Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Chapter 18 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
Ch. 18.1 - Prob. 18.1QCCh. 18.4 - Problem 19-2 Complete the equation for each...Ch. 18.4 - Prob. 18.3QCCh. 18 - Prob. 1PCh. 18 - Write the IUPAC name for each compound.Ch. 18 - Prob. 3PCh. 18 - Prob. 4PCh. 18 - Prob. 5PCh. 18 - Prob. 6PCh. 18 - 0 Complete the equations for these reactions.
Ch. 18 - Prob. 8PCh. 18 - Prob. 9PCh. 18 - Prob. 10PCh. 18 - Prob. 11PCh. 18 - Prob. 12PCh. 18 - 6 Why are Dacron and Mylar referred to as...Ch. 18 - 7 What type of structural feature do the...Ch. 18 - Prob. 15PCh. 18 - Prob. 16PCh. 18 - 0 Show how triphosphoric acid can form from three...Ch. 18 - 1 Write an equation for the hydrolysis of...Ch. 18 - Prob. 19PCh. 18 - (The Pyrethrins-Natural Insecticides of Plant...Ch. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - Prob. 24PCh. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - Prob. 34PCh. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - Prob. 40PCh. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Prob. 44PCh. 18 - Prob. 45PCh. 18 - Prob. 46P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY