
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
10th Edition
ISBN: 9781305957510
Author: ZUMDAHL, Steven S.; Zumdahl, Susan A.; DeCoste, Donald J.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 112AE
Consider the following half-reactions:
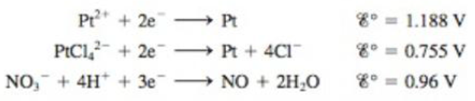
Explain why platinum metal will dissolve in aqua regia (a mixture of hydrochloric and nitric acids) but not in either concentrated nitric or concentrated hydrochloric acid individually.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Synthesize the following:
Did you report your data to the correct number of significant
figures?
Temperature of cold water (°C)
4.0
Temperature of hot water ("C)
87.0
Volume of cold water (mL)
94.0
Volume of hot water (mL)
78.0
Final temperature after mixing ("C)
41.0
Mass of cold water (g)
94.0
Mass of hot water (g)
78.0
Calorimeter constant (J/°C)
12.44
How to calculate the calorimeter constant
please draw the arrows
Chapter 18 Solutions
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
Ch. 18 - What is a half-reaction? Why must the number of...Ch. 18 - Galvanic cells harness spontaneous...Ch. 18 - Table 17-1 lists common half-reactions along with...Ch. 18 - Consider the equation G = -nF. What are the four...Ch. 18 - The Nernst equation allows determination of the...Ch. 18 - What are concentration cells? What is in a...Ch. 18 - Prob. 7RQCh. 18 - Prob. 8RQCh. 18 - What characterizes an electrolytic cell? What is...Ch. 18 - Prob. 1ALQ
Ch. 18 - When balancing reactions in Chapter 3, we did not...Ch. 18 - Sketch a galvanic cell, and explain how it works....Ch. 18 - In making a specific galvanic cell, explain how...Ch. 18 - Prob. 5ALQCh. 18 - Prob. 6ALQCh. 18 - Sketch a cell that forms iron metal from iron(II)...Ch. 18 - Which of the following is the best reducing agent:...Ch. 18 - You are told that metal A is a better reducing...Ch. 18 - Explain the following relationships: G and w, cell...Ch. 18 - Explain why cell potentials are not multiplied by...Ch. 18 - What is the difference between and ? When is equal...Ch. 18 - Consider the following galvanic cell: What happens...Ch. 18 - Look up the reduction potential for Fe3+ to Fe2+....Ch. 18 - If the cell potential is proportional to work and...Ch. 18 - Is the following statement true or false?...Ch. 18 - Define oxidation and reduction in terms of both...Ch. 18 - Assign oxidation numbers to all the atoms in each...Ch. 18 - Specify which of the following equations represent...Ch. 18 - The Ostwald process for the commercial production...Ch. 18 - Balance the following oxidation-reduction...Ch. 18 - Balance the following oxidation-reduction...Ch. 18 - What is electrochemistry? What are redox...Ch. 18 - Prob. 24QCh. 18 - When magnesium metal is added to a beaker of...Ch. 18 - How can one construct a galvanic cell from two...Ch. 18 - The free energy change for a reaction, G, is an...Ch. 18 - What is wrong with the following statement: The...Ch. 18 - When jump-starting a car with a dead battery, the...Ch. 18 - In theory, most metals should easily corrode in...Ch. 18 - Consider the electrolysis of a molten salt of some...Ch. 18 - Consider the following electrochemical cell: a. If...Ch. 18 - Prob. 33QCh. 18 - Prob. 34QCh. 18 - Consider the following galvanic cell: Label the...Ch. 18 - Consider the following galvanic cell: a. Label the...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Consider the following galvanic cells: For each...Ch. 18 - Give the balanced cell equation and determine for...Ch. 18 - Calculate values for the following g cells. Which...Ch. 18 - Calculate values for the following cells. Which...Ch. 18 - Chlorine dioxide (C1O2), which is produced by the...Ch. 18 - The amount of manganese in steel is determined by...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Estimate for the half-reaction 2H2O+2eH2+2OH given...Ch. 18 - The equation G = nF also can be applied to...Ch. 18 - Glucose is the major fuel for most living cells....Ch. 18 - Direct methanol fuel cells (DMFCs) have shown some...Ch. 18 - Using data from Table 17-1, place the following in...Ch. 18 - Using data from Table 17-1, place the following in...Ch. 18 - Answer the following questions using data from...Ch. 18 - Answer the following questions using data from...Ch. 18 - Consider only the species (at standard conditions)...Ch. 18 - Prob. 62ECh. 18 - Use the table of standard reduction potentials...Ch. 18 - Consider the concentration cell in Fig. 17-10. If...Ch. 18 - Consider the concentration cell shown below....Ch. 18 - Consider a concentration cell similar to the one...Ch. 18 - The overall reaction in the lead storage battery...Ch. 18 - Calculate the pH of the cathode compartment for...Ch. 18 - Consider the cell described below:...Ch. 18 - Consider the cell described below:...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a standard...Ch. 18 - Prob. 78ECh. 18 - An electrochemical cell consists of a standard...Ch. 18 - An electrochemical cell consists of a nickel metal...Ch. 18 - Consider a concentration cell that has both...Ch. 18 - You have a concentration cell in which the cathode...Ch. 18 - Under standard conditions, what reaction occurs,...Ch. 18 - A disproportionation reaction involves a substance...Ch. 18 - Consider the following galvanic cell at 25C:...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - Cadmium sulfide is used in some semiconductor...Ch. 18 - For the following half-reaction, = 2.07 V:...Ch. 18 - Calculate for the following half-reaction:...Ch. 18 - The solubility product for CuI(s) is 1.1 102...Ch. 18 - How long will it take to plate out each of the...Ch. 18 - The electrolysis of BiO+ produces pure bismuth....Ch. 18 - What mass of each of the following substances can...Ch. 18 - Aluminum is produced commercially by the...Ch. 18 - Electrolysis of an alkaline earth metal chloride...Ch. 18 - What volume of F2 gas, at 25C and 1.00 atm, is...Ch. 18 - What volumes of H2(g) and O2(g) at STP are...Ch. 18 - A single HallHeroult cell (as shown in Fig. 17-22)...Ch. 18 - A factory wants to produce 1.00 103 kg barium...Ch. 18 - It took 2.30 min using a current of 2.00 A to...Ch. 18 - A solution containing Pt4+ is electrolyzed with a...Ch. 18 - A solution at 25C contains 1.0 M Cd2+, 1.0 M Ag+,...Ch. 18 - A solution at 25C contains 1.0 M Cu2 and 1.0 104...Ch. 18 - In the electrolysis of an aqueous solution of...Ch. 18 - Copper can be plated onto a spoon by placing the...Ch. 18 - Prob. 107ECh. 18 - Prob. 108ECh. 18 - What reactions take place at the cathode and the...Ch. 18 - What reaction will take place at the Cathode and...Ch. 18 - The saturated calomel electrode. abbreviated SCE....Ch. 18 - Consider the following half-reactions: Explain why...Ch. 18 - Consider the standard galvanic cell based on the...Ch. 18 - A standard galvanic cell is constructed so that...Ch. 18 - The black silver sulfide discoloration of...Ch. 18 - Prob. 116AECh. 18 - When aluminum foil is placed in hydrochloric acid,...Ch. 18 - Prob. 118AECh. 18 - Prob. 119AECh. 18 - Prob. 120AECh. 18 - A fuel cell designed to react grain alcohol with...Ch. 18 - The overall reaction and equilibrium constant...Ch. 18 - Prob. 123AECh. 18 - The overall reaction and standard cell potential...Ch. 18 - Prob. 125AECh. 18 - The ultimate electron acceptor in the respiration...Ch. 18 - One of the few industrial-scale processes that...Ch. 18 - It took 150. s for a current of 1.25 A to plate...Ch. 18 - Prob. 129AECh. 18 - In the electrolysis of a sodium chloride solution,...Ch. 18 - An aqueous solution of an unknown salt of...Ch. 18 - Which of the following statement(s) is/are true?...Ch. 18 - Consider a galvanic cell based on the following...Ch. 18 - Prob. 134CWPCh. 18 - Consider a galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - An aqueous solution of PdCl2 is electrolyzed for...Ch. 18 - Consider the following half-reactions:...Ch. 18 - Consider the following reduction potentials: Co3++...Ch. 18 - Calculate and G for the reaction 2H2O(l) 2H2(g)...Ch. 18 - Prob. 141CPCh. 18 - The overall reaction in the lead storage battery...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - A zinc-copper battery is constructed at follows at...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Consider a cell based on the following...Ch. 18 - Prob. 147CPCh. 18 - You have a concentration cell with Cu electrodes...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Given the following two standard reduction...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - Prob. 152CPCh. 18 - Consider the following galvanic cell: A 15 0-mole...Ch. 18 - When copper reacts with nitric acid, a mixture of...Ch. 18 - The following standard reduction potentials have...Ch. 18 - An electrochemical cell is set up using the...Ch. 18 - Three electrochemical cells were connected in...Ch. 18 - A silver concentration cell is set up at 25C as...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - The table below lists the cell potentials for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forwardQuestion 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forward
- Identify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY