
Chemistry with Access Code, Hybrid Edition
9th Edition
ISBN: 9781285188492
Author: Steven S. Zumdahl
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 10ALQ
What is the difference between 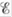 and
and 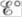 ? When is
? When is 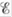 equal to zero? When is
equal to zero? When is 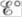 equal to zero? (Consider “regular” galvanic cells as well as concentration cells.)
equal to zero? (Consider “regular” galvanic cells as well as concentration cells.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculating the pH at equivalence of a titration
Try Again
Your answer is incorrect.
0/5
a
A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of
hydrocyanic acid is 9.21.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added.
pH
=
11.43]
G
00.
18
Ar
B•
Biological Macromolecules
Naming and drawing the products of aldose oxidation and reduction
aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions.
Click and drag to start drawing a
structure.
X
AP
‡
1/5
Naor
Explanation
Check
McGraw Hill LLC. All Rights Reserved. Terms of Use
Privacy Center
Accessibil
● Biological Macromolecules
Identifying the parts of a disaccharide
Take a look at this molecule, and then answer the questions in the table below it.
CH2OH
O
H
H
H
OH
OH
OH
H
H
CH2OH
H
O
OH
H
OH H
H
H
H
OH
Is this a reducing sugar?
Does this molecule contain a glycosidic bond?
If you said this molecule does contain a glycosidic bond, write the symbol
describing it.
If you said this molecule does contain a glycosidic bond, write the common
names (including anomer and enantiomer labels) of the molecules that
would be released if that bond were hydrolyzed.
If there's more than one molecule, separate each name with a comma.
Explanation
Check
O yes
X
O no
○ yes
O no
U
Chapter 18 Solutions
Chemistry with Access Code, Hybrid Edition
Ch. 18 - What is a half-reaction? Why must the number of...Ch. 18 - Galvanic cells harness spontaneous...Ch. 18 - Table 17-1 lists common half-reactions along with...Ch. 18 - Consider the equation G = -nF. What are the four...Ch. 18 - The Nernst equation allows determination of the...Ch. 18 - What are concentration cells? What is in a...Ch. 18 - Prob. 7RQCh. 18 - Prob. 8RQCh. 18 - What characterizes an electrolytic cell? What is...Ch. 18 - Sketch a galvanic cell, and explain how it works....
Ch. 18 - In making a specific galvanic cell, explain how...Ch. 18 - Prob. 3ALQCh. 18 - Prob. 4ALQCh. 18 - Sketch a cell that forms iron metal from iron(II)...Ch. 18 - Which of the following is the best reducing agent:...Ch. 18 - You are told that metal A is a better reducing...Ch. 18 - Explain the following relationships: G and w, cell...Ch. 18 - Explain why cell potentials are not multiplied by...Ch. 18 - What is the difference between and ? When is equal...Ch. 18 - Consider the following galvanic cell: What happens...Ch. 18 - Look up the reduction potential for Fe3+ to Fe2+....Ch. 18 - If the cell potential is proportional to work and...Ch. 18 - Is the following statement true or false?...Ch. 18 - Define oxidation and reduction in terms of both...Ch. 18 - Assign oxidation numbers to all the atoms in each...Ch. 18 - Specify which of the following equations represent...Ch. 18 - The Ostwald process for the commercial production...Ch. 18 - What is electrochemistry? What are redox...Ch. 18 - When balancing equations in Chapter 3, we did not...Ch. 18 - When magnesium metal is added to a beaker of...Ch. 18 - How can one construct a galvanic cell from two...Ch. 18 - The free energy change for a reaction, G, is an...Ch. 18 - What is wrong with the following statement: The...Ch. 18 - When jump-starting a car with a dead battery, the...Ch. 18 - In theory, most metals should easily corrode in...Ch. 18 - Consider the electrolysis of a molten salt of some...Ch. 18 - Consider the following electrochemical cell: a. If...Ch. 18 - Prob. 29ECh. 18 - Prob. 30ECh. 18 - Balance the following oxidationreduction reactions...Ch. 18 - Balance the following oxidationreduction reactions...Ch. 18 - Chlorine gas was first prepared in 1774 by C. W....Ch. 18 - Prob. 34ECh. 18 - Consider the following galvanic cell: Label the...Ch. 18 - Consider the following galvanic cell: a. Label the...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Calculate values for the galvanic cells in...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Sketch the galvanic cells based on the following...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Give the standard line notation for each cell in...Ch. 18 - Consider the following galvanic cells: For each...Ch. 18 - Give the balanced cell equation and determine for...Ch. 18 - Calculate values for the following g cells. Which...Ch. 18 - Calculate values for the following cells. Which...Ch. 18 - Chlorine dioxide (C1O2), which is produced by the...Ch. 18 - The amount of manganese in steel is determined by...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Calculate the maximum amount of work that can be...Ch. 18 - Estimate for the half-reaction 2H2O+2eH2+2OH given...Ch. 18 - The equation G = nF also can be applied to...Ch. 18 - Glucose is the major fuel for most living cells....Ch. 18 - Direct methanol fuel cells (DMFCs) have shown some...Ch. 18 - Using data from Table 18.1, place the following in...Ch. 18 - Using data from Table 18.1, place the following in...Ch. 18 - Answer the following questions using data from...Ch. 18 - Answer the following questions using data from...Ch. 18 - Consider only the species (at standard conditions)...Ch. 18 - Prob. 62ECh. 18 - Use the table of standard reduction potentials...Ch. 18 - Use the table of standard reduction potentials...Ch. 18 - Prob. 65ECh. 18 - Prob. 66ECh. 18 - Consider the concentration cell in Fig. 17-10. If...Ch. 18 - Consider the concentration cell shown below....Ch. 18 - Consider a concentration cell similar to the one...Ch. 18 - The overall reaction in the lead storage battery...Ch. 18 - Calculate the pH of the cathode compartment for...Ch. 18 - Consider the cell described below:...Ch. 18 - Consider the cell described below:...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Calculate G and K at 25C for the reactions in...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - Consider the galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a standard...Ch. 18 - Prob. 80ECh. 18 - An electrochemical cell consists of a standard...Ch. 18 - An electrochemical cell consists of a nickel metal...Ch. 18 - Consider a concentration cell that has both...Ch. 18 - You have a concentration cell in which the cathode...Ch. 18 - Under standard conditions, what reaction occurs,...Ch. 18 - A disproportionation reaction involves a substance...Ch. 18 - Consider the following galvanic cell at 25C:...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - Prob. 89ECh. 18 - For the following half-reaction, = 2.07 V:...Ch. 18 - Calculate for the following half-reaction:...Ch. 18 - The solubility product for CuI(s) is 1.1 102...Ch. 18 - How long will it take to plate out each of the...Ch. 18 - The electrolysis of BiO+ produces pure bismuth....Ch. 18 - What mass of each of the following substances can...Ch. 18 - Aluminum is produced commercially by the...Ch. 18 - An unknown metal M is electrolyzed. It took 74.1 s...Ch. 18 - Prob. 98ECh. 18 - What volume of F2 gas, at 25C and 1.00 atm, is...Ch. 18 - What volumes of H2(g) and O2(g) at STP are...Ch. 18 - A single HallHeroult cell (as shown in Fig. 17-22)...Ch. 18 - A factory wants to produce 1.00 103 kg barium...Ch. 18 - It took 2.30 min using a current of 2.00 A to...Ch. 18 - A solution containing Pt4+ is electrolyzed with a...Ch. 18 - A solution at 25C contains 1.0 M Cd2+, 1.0 M Ag+,...Ch. 18 - Prob. 106ECh. 18 - In the electrolysis of an aqueous solution of...Ch. 18 - Copper can be plated onto a spoon by placing the...Ch. 18 - Prob. 109ECh. 18 - Prob. 110ECh. 18 - What reactions take place at the cathode and the...Ch. 18 - What reaction will take place at the Cathode and...Ch. 18 - Gold is produced electrochemically from an aqueous...Ch. 18 - The blood alcohol (C2H5OH) level can be determined...Ch. 18 - The saturated calomel electrode. abbreviated SCE....Ch. 18 - Consider the following half-reactions: Explain why...Ch. 18 - Consider the standard galvanic cell based on the...Ch. 18 - A standard galvanic cell is constructed so that...Ch. 18 - The black silver sulfide discoloration of...Ch. 18 - Prob. 120AECh. 18 - When aluminum foil is placed in hydrochloric acid,...Ch. 18 - Prob. 122AECh. 18 - A fuel cell designed to react grain alcohol with...Ch. 18 - The overall reaction and equilibrium constant...Ch. 18 - Prob. 125AECh. 18 - The overall reaction and standard cell potential...Ch. 18 - Prob. 127AECh. 18 - The ultimate electron acceptor in the respiration...Ch. 18 - One of the few industrial-scale processes that...Ch. 18 - It took 150. s for a current of 1.25 A to plate...Ch. 18 - Prob. 131AECh. 18 - In the electrolysis of a sodium chloride solution,...Ch. 18 - An aqueous solution of an unknown salt of...Ch. 18 - Which of the following statement(s) is/are true?...Ch. 18 - Consider a galvanic cell based on the following...Ch. 18 - Prob. 136CWPCh. 18 - Consider a galvanic cell based on the following...Ch. 18 - An electrochemical cell consists of a silver metal...Ch. 18 - An aqueous solution of PdCl2 is electrolyzed for...Ch. 18 - Prob. 140CPCh. 18 - Prob. 141CPCh. 18 - The overall reaction in the lead storage battery...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - A zinc-copper battery is constructed at follows at...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Consider a cell based on the following...Ch. 18 - Prob. 147CPCh. 18 - You have a concentration cell with Cu electrodes...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - Given the following two standard reduction...Ch. 18 - Consider the following galvanic cell: Calculate...Ch. 18 - Prob. 152CPCh. 18 - Consider the following galvanic cell: A 15 0-mole...Ch. 18 - When copper reacts with nitric acid, a mixture of...Ch. 18 - The following standard reduction potentials have...Ch. 18 - An electrochemical cell is set up using the...Ch. 18 - Three electrochemical cells were connected in...Ch. 18 - A silver concentration cell is set up at 25C as...Ch. 18 - A galvanic cell is based on the following...Ch. 18 - The table below lists the cell potentials for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Give the IUPAC name for each compound.
Organic Chemistry
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forward
- Draw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward
- (2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax  General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY