
Concept explainers
(a)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
First step is the formation of more stable carbocation which is followed by the attack of nucleophile. Formation of more stable carbocation and the leaving group present in the substrate plays very important role in the reactivity of
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(b)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Elimination reaction of an
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(c)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Structure of the substrate plays major role in the reactivity of
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(d)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
First step is the formation of more stable carbocation which is followed by the attack of nucleophile. Formation of more stable carbocation and the leaving group present in the substrate plays very important role in the reactivity of
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(e)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Ozonolysis:
Alkene reacts with ozone which cleaves the double bond followed by work up with dimethyl sulfide forms
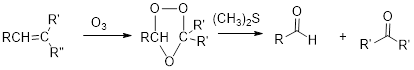
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Elimination reaction of an alkyl halide results in the formation of an alkene.
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(f)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Ozonolysis:
Alkene reacts with ozone which cleaves the double bond followed by work up with dimethyl sulfide forms ketone.
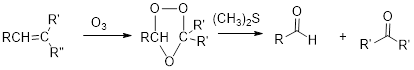
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as
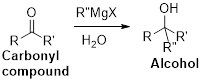
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry 3rd.ed. Klein Evaluation/desk Copy
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Identifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAssign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





