
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 83E
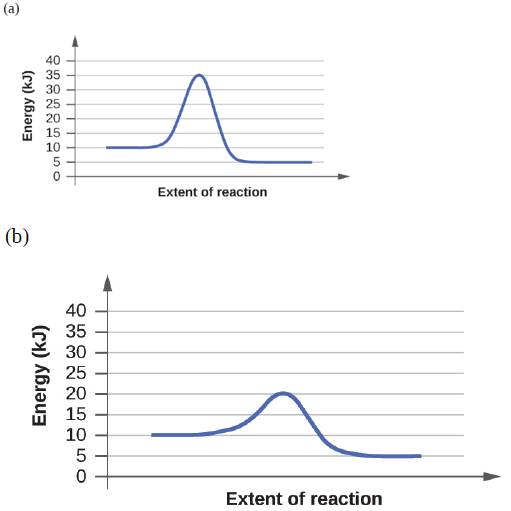
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
cold
KMnO4, NaOH
2. DMS
1. 03
CH3OH
Br2
1.
03
2. (CH3)2S
H₂
Pd or Pt (catalyst)
HBr
18
19
20 1
HBr
ROOR (peroxide)
H₂O
H₂SO4
HCI
HI
17
16
6
15
MCPBA
1. BH3 THF
2. H₂O2, NaOH
1. OsO4
2. H₂O₂
110
CH3CO₂H
(peroxyacid)
1. MCPBA
2. H₂O*
Br2
H₂O
BH3 THF
B12
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
Bra
A
B
C
D
H
OH
H
OH
OH
H
OH
α α α
OH
H
OH
OH
фон
d
H
"H
Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.
Electrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.
Chapter 17 Solutions
Chemistry Atoms First2e
Ch. 17 - What is the difference between average rate,...Ch. 17 - Ozone decomposes to oxygen according to the...Ch. 17 - In the nuclear industry, chlorine trifluoride is...Ch. 17 - A study of the rate of dimerization of C4H6 gave...Ch. 17 - A study of the rate of the reaction represented as...Ch. 17 - Consider the following reaction in aqueous...Ch. 17 - Describe the effect of each of the following on...Ch. 17 - Explain why an egg cooks move slowly in boiling...Ch. 17 - Go to the PhET Reactions and change to Angled...Ch. 17 - In the PhET Reactions tab to observe how multiple...
Ch. 17 - In the PhET Reactions under Options. (a) Leave...Ch. 17 - How do the rate of a reaction and its rate...Ch. 17 - Doubling the concentration of a reactant increases...Ch. 17 - Tripling the concentration of a reactant increases...Ch. 17 - How much and in what direction will each of the...Ch. 17 - How will each of the following affect the rate of...Ch. 17 - Regular ?ights of supersonic aircraft in the...Ch. 17 - Radioactive phosphorus is used in the study of...Ch. 17 - The rate constant for the radioactive decay of 14C...Ch. 17 - The decomposition of acetaldehyde is a second...Ch. 17 - Alcohol is removed from the bloodstream by a...Ch. 17 - Under certain conditions the decomposition of...Ch. 17 - Nitrosyl chloride, NOCI, decomposes to NO and CI2....Ch. 17 - From the following data, determine the rate...Ch. 17 - Nitrogen monoxide reacts with chlorine according...Ch. 17 - Hydrogen reacts with nitrogen monoxide to form...Ch. 17 - For the reaction AB+C, the following data were...Ch. 17 - For the reaction QW+X, the following data were...Ch. 17 - The rate constant for the ?rst-order decomposition...Ch. 17 - The annual production of HNO3 in 2013 was 60...Ch. 17 - The following data have been determined for the...Ch. 17 - Describe how graphical methods can be used to...Ch. 17 - Use the data provided to graphically determine the...Ch. 17 - Pure ozone decomposes slowly to oxygen, 2O33O2(g)....Ch. 17 - From the given data, use a graphical method to...Ch. 17 - What is the half-life for the first-order decay of...Ch. 17 - What is the half-life for the first-order decay of...Ch. 17 - What is the half-life for the decomposition of...Ch. 17 - What is the half-life for the decomposition of O3...Ch. 17 - The reaction of compoundA to give compoundsC andD...Ch. 17 - The half-life of a reaction of compoundA to give...Ch. 17 - Some bacteria are resistant to the antibiotic...Ch. 17 - Both technetium-99 and thallium-201 are used to...Ch. 17 - There are two molecules with the formula C3H6...Ch. 17 - Fluorine-18 is a radioactive isotope that decays...Ch. 17 - Suppose that the half-life of steroids taken by an...Ch. 17 - Recently, the skeleton of King Richard III was...Ch. 17 - Nitroglycerine is an extremely sensitive...Ch. 17 - For the past 10 years, the unsaturated hydrocarbon...Ch. 17 - Chemical reactions occur when reactants collide....Ch. 17 - When every collision between reactants leads to a...Ch. 17 - What is the activation energy of a reaction, and...Ch. 17 - Account for the relationship between the rate of a...Ch. 17 - Describe how graphical methods can be used to...Ch. 17 - How does an increase in temperature affect rate of...Ch. 17 - The rate of a certain reaction doubles for every...Ch. 17 - In an experiment, a sample of NaClO3 was 90%...Ch. 17 - The rate constant at 325 C for the decomposition...Ch. 17 - The rate constant for the decomposition of...Ch. 17 - An elevated level of the enzyme alkaline...Ch. 17 - In terms of collision theory, to which of the...Ch. 17 - Hydrogen iodide, HI, decomposes in the gas phase...Ch. 17 - The element Co exists in two oxidation states,...Ch. 17 - The hydrolysis of the sugar sucrose to the sugars...Ch. 17 - Use the PhET Reactions Single collision" tab of...Ch. 17 - Use the PhET Reactions Single collision tab of the...Ch. 17 - Why awe elementary reactions involving three or...Ch. 17 - In general, can we predict the effect of doubling...Ch. 17 - Define these terms: (a) unimolecular reaction (b)...Ch. 17 - What is the rate law for the elementary...Ch. 17 - Given the following reactions and the...Ch. 17 - Write the rate equation for each of the following...Ch. 17 - Nitrogen (Il) oxide, NO, reacts with hydrogen, H2,...Ch. 17 - Experiments were conducted to study the rate of...Ch. 17 - The reaction of CO with CI2 gives phosgene...Ch. 17 - . Account for the increase in reaction rate...Ch. 17 - Compare the functions of homogeneous and...Ch. 17 - Consider this scenario and answer the following...Ch. 17 - Water gas is a 1:1 mixture of carbon monoxide and...Ch. 17 - Nitrogen and oxygen react at high temperatures....Ch. 17 - For each of the following pairs of reaction...Ch. 17 - For each of the following pairs of reaction...Ch. 17 - For each of the following reaction diagrams,...Ch. 17 - For each of the following reaction diagrams,...Ch. 17 - Assuming the diagrams in Exercise 17.83 represent...Ch. 17 - Consider the similarities and differences in the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Refer to figure 10.2 to find the electronegativity different between each of elements; then refer to Table 10.2...
Introductory Chemistry (6th Edition)
WRITE ABOUT A THEME: INTERACTIONS Animal life changed greatly during the Cambrian explosion, with some groups e...
Campbell Biology (11th Edition)
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
Your bore cells, muscle cells, and skin cells look different because a. different kinds of genes are present in...
Campbell Essential Biology (7th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forwardGiven the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY