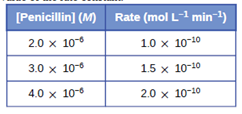
Problem 1E: What is the difference between average rate, initial rate, and instantaneous rate? Problem 2E: Ozone decomposes to oxygen according to the equation 2O3(g)3O2(g). Write the equation that relates... Problem 3E: In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile... Problem 4E: A study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a)... Problem 5E: A study of the rate of the reaction represented as 2AB gave the following data: Time (s) 0.0 5.0... Problem 6E: Consider the following reaction in aqueous solution: 5Br-(aq)+BrO3(aq)+6H+(aq)3Br2(aq)+3H2O(l) If... Problem 7E: Describe the effect of each of the following on the rate of the reaction of magnesium metal with a... Problem 8E: Explain why an egg cooks move slowly in boiling water in Denver than in New York City. (Hint:... Problem 9E: Go to the PhET Reactions and change to Angled shot to see the difference. (a) What happens when the... Problem 10E: In the PhET Reactions tab to observe how multiple atoms and molecules interact under varying... Problem 11E: In the PhET Reactions under Options. (a) Leave the Initial Temperature at the default setting.... Problem 12E: How do the rate of a reaction and its rate constant differ? Problem 13E: Doubling the concentration of a reactant increases the rate of a reaction four times. With this... Problem 14E: Tripling the concentration of a reactant increases the rate of a reaction nine times. With this... Problem 15E: How much and in what direction will each of the following affect the rate of the reaction:... Problem 16E: How will each of the following affect the rate of the reaction: CO(g)+NO2CO2(g)+NO(g) } if the rate... Problem 17E: Regular ?ights of supersonic aircraft in the stratosphere ale of concern because such aircraft... Problem 18E: Radioactive phosphorus is used in the study of biochemical reaction mechanisms because phosphorus... Problem 19E: The rate constant for the radioactive decay of 14C is 1.21104 year-1. The products of the decay are... Problem 20E: The decomposition of acetaldehyde is a second order reaction with a rate constant of 4.71108... Problem 21E: Alcohol is removed from the bloodstream by a series of metabolic reactions. The first reaction... Problem 22E: Under certain conditions the decomposition of ammonia on a metal surface gives the following data:... Problem 23E: Nitrosyl chloride, NOCI, decomposes to NO and CI2. 2NOCI(g)2NO(g)+CI2(g) Determine the rate... Problem 24E: From the following data, determine the rate equation, the rate constant, and the order with respect... Problem 25E: Nitrogen monoxide reacts with chlorine according to the equation: 2NOCI(g)2NOCI(g) The following... Problem 26E: Hydrogen reacts with nitrogen monoxide to form dinitrogen monoxide (laughing gas) according to the... Problem 27E: For the reaction AB+C, the following data were obtained at 30 C: [A] (M) 0.230 0.356 0.557 Rate... Problem 28E: For the reaction QW+X, the following data were obtained at 30 C: [Q]initial (M) 0.170 0.212 0.357... Problem 29E: The rate constant for the ?rst-order decomposition at 45 C of dinitrogen pentoxide, N2O5, dissolved... Problem 30E: The annual production of HNO3 in 2013 was 60 million metric tons Most of that was prepared by the... Problem 31E: The following data have been determined for the reaction: I+OCIIO+CI 1 2 3 [I] 0.10 0.20 0.30 [OCI]... Problem 32E: Describe how graphical methods can be used to determine the order of a reaction and its rate... Problem 33E: Use the data provided to graphically determine the order and rate constant of the following... Problem 34E: Pure ozone decomposes slowly to oxygen, 2O33O2(g). Use the data provided in a graphical method and... Problem 35E: From the given data, use a graphical method to determine the order and rate constant of the... Problem 36E: What is the half-life for the first-order decay of phosphorus-32? (P1532S1632+e) The rate constant... Problem 37E: What is the half-life for the first-order decay of carbon-14? (C614N714+e) The rate constant for the... Problem 38E: What is the half-life for the decomposition of NOCl when the concentration of NOCl is 0.15M ? The... Problem 39E: What is the half-life for the decomposition of O3 when the concentration of O3is2.3510-6M? The rate... Problem 40E: The reaction of compoundA to give compoundsC andD was found to be second-order inA. The rate... Problem 41E: The half-life of a reaction of compoundA to give compoundsD andE is 8.50 min when the initial... Problem 42E: Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an... Problem 43E: Both technetium-99 and thallium-201 are used to image heart muscle in patients with suspected heart... Problem 44E: There are two molecules with the formula C3H6 Propane, CH3CH = CH2, is the monomer of the polymer... Problem 45E: Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a... Problem 46E: Suppose that the half-life of steroids taken by an athlete is 42 days. Assuming that the steroids... Problem 47E: Recently, the skeleton of King Richard III was found under a parking lot in England. If tissue... Problem 48E: Nitroglycerine is an extremely sensitive explosive. In a series of carefully controlled experiments,... Problem 49E: For the past 10 years, the unsaturated hydrocarbon 1, 3-butadiene (CH2 = CH - CH = CH2) has ranked... Problem 50E: Chemical reactions occur when reactants collide. What are two factors that may prevent a collision... Problem 51E: When every collision between reactants leads to a reaction, what determines the rate at which the... Problem 52E: What is the activation energy of a reaction, and how is this energy related to the activated complex... Problem 53E: Account for the relationship between the rate of a reaction and its activation energy. Problem 54E: Describe how graphical methods can be used to determine the activation energy of a reaction from a... Problem 55E: How does an increase in temperature affect rate of reaction? Explain this effect in terms of the... Problem 56E: The rate of a certain reaction doubles for every 10 C rise in temperature. (a) How much faster does... Problem 57E: In an experiment, a sample of NaClO3 was 90% decomposed in 48 min. Approximately how long would this... Problem 58E: The rate constant at 325 C for the decomposition reaction C4H82C2H4 is 6.1108s1, and the activation... Problem 59E: The rate constant for the decomposition of acetaldehyde, CH3CHO, to methane, CH4, and carbon... Problem 60E: An elevated level of the enzyme alkaline phosphatase (ALP) in human serum is an indication of... Problem 61E: In terms of collision theory, to which of the following is the rate of a chemical reaction... Problem 62E: Hydrogen iodide, HI, decomposes in the gas phase to produce hydrogen, H2, and iodine, I2. The value... Problem 63E: The element Co exists in two oxidation states, Co(II) and Co(III), and the ions form many complexes.... Problem 64E: The hydrolysis of the sugar sucrose to the sugars glucose and fructose, C12H22O11+H2OC6H12O6+C6H12O6... Problem 65E: Use the PhET Reactions Single collision" tab of the simulation applet, enable the Energy view by... Problem 66E: Use the PhET Reactions Single collision tab of the simulation applet, enable the Energy view" by... Problem 67E: Why awe elementary reactions involving three or more reactants very uncommon? Problem 68E: In general, can we predict the effect of doubling the concentration of A on the rate of the overall... Problem 69E: Define these terms: (a) unimolecular reaction (b) bimolecular reaction (c) elementary reaction (d)... Problem 70E: What is the rate law for the elementary termolecular reaction A+2B products? For 3A products? Problem 71E: Given the following reactions and the corresponding rate laws, in which of the reactions might the... Problem 72E: Write the rate equation for each of the following elementary reactions: (a) O3sunlightO2+O (b)... Problem 73E: Nitrogen (Il) oxide, NO, reacts with hydrogen, H2, according to the following equation:... Problem 74E: Experiments were conducted to study the rate of the reaction represented by this equation.[3]... Problem 75E: The reaction of CO with CI2 gives phosgene (COCI2), a nerve gas that was used in World War I. Use... Problem 76E: . Account for the increase in reaction rate brought about by a catalyst. Problem 77E: Compare the functions of homogeneous and heterogeneous catalysts. Problem 78E: Consider this scenario and answer the following questions: Chlorine atoms resulting from... Problem 79E: Water gas is a 1:1 mixture of carbon monoxide and hydrogen gas and is called water gas because it is... Problem 80E: Nitrogen and oxygen react at high temperatures. What will happen to the concentrations of... Problem 81E: For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed: Problem 82E: For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed: Problem 83E: For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction: Problem 84E: For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction: Problem 85E: Assuming the diagrams in Exercise 17.83 represent different mechanisms for the same reaction, which... Problem 86E: Consider the similarities and differences in the two reaction diagrams shown in Exercise 17.84. Do... format_list_bulleted


 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning




