
Concept explainers
Epoxide Rearrangements and the NIH Shift
This passage is about two seemingly unrelated aspects of
epoxide rearrangements
arene oxides
These two topics merge in an important biological transformation in which neither the
reactant nor the product is an epoxide

Epoxide rearrangements
In some epoxide ring-opening reactions
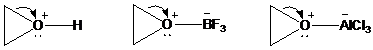
As positive charge develops on the ring carbon, one of the groups on the adjacent carbon migrates to it. This migration is assisted by electron
all of this occurs in the same transition state. Subsequent deprotonation gives an
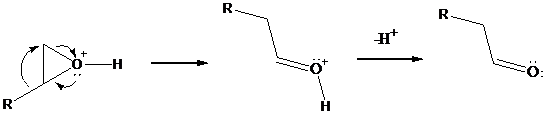
Overall, the reaction resembles the pinacol rearrangement of vicinal
Descriptive Passage and Interpretive Problems) and takes place under similar conditions.
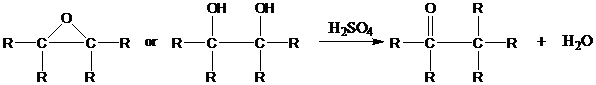
Arene Oxides
Aromatic rings are normally inert to the customary reagents that convert
arene oxides have been synthesized in the laboratory, often by indirect methods. Their chemical
reactivity resembles that of other epoxides.

The most striking thing about arene oxides is their involvement in biological processes. Enzymes
In the liver oxidize
The NIH shift
Although hydroxylation of phenylalanine to tyrosine looks like a typical electrophilic aromatic sub stitution, scientists at the U.S. National Institutes of Health discovered that the biochemical pathway combines epoxidation of the benzene ring followed by epoxide ring opening with rearrangement. This rearrangement, which is the biochemical analog of the pinacol

Acetanilide, which has pain-relieving properties, undergoes a biochemical oxidation
similar to that of the NIH shift that occurs with phenylalanine. The product formed from
acetanilide is itself a pain reliever. What is the structure of this substance (better known as
Tylenol)?
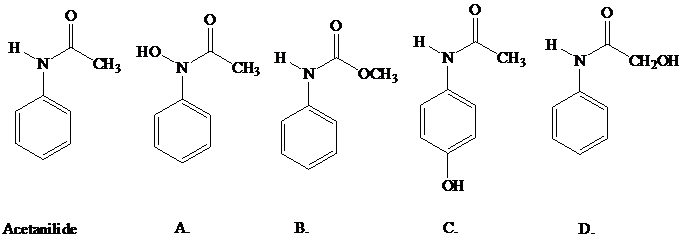
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- NH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forward
- Intercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forwardIndicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

