
Concept explainers
(a)
Interpretation: The structure of given compounds has to be drawn.
ethylacetoacetate
Concept introduction: The
Carboxylate ion with a carbonyl group at the 3-position loses
(a)
Answer to Problem 48P
The structure of ethyl acetoacetae is,
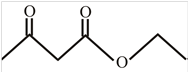
Explanation of Solution
The name given compound ethyl acetoacetate ends with ate. This means the given compound must contain an ester group. The name of compound starts with ethyl group, this means ethyl group is attached to the oxygen of an ester group. The acetone group is attached with the carbonyl carbon of an ester group. The structure of ethyl acetoacetae is,

(b)
Interpretation: The structure of given compounds has to be drawn.
α-methylmalonicacid
Concept introduction: The
Carboxylate ion with a carbonyl group at the 3-position loses
(b)
Answer to Problem 48P
The structure of α-methylmalonic acid is,
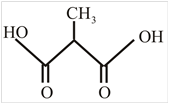
Explanation of Solution
The structure of malonic acid is two

(c)
Interpretation: The structure of given compounds has to be drawn.
β-keto ester
Concept introduction: The
Carboxylate ion with a carbonyl group at the 3-position loses
(c)
Answer to Problem 48P
The structure of β-keto ester is,

Explanation of Solution
The given compound β-keto ester contains a keto group and an ester group. The structure of β-keto ester is,

(d)
Interpretation: The structure of given compounds has to be drawn.
The carboxylic acid obtained from malonic ester synthesis when the
Concept introduction: The
Carboxylate ion with a carbonyl group at the 3-position loses
(d)
Answer to Problem 48P
The structure of enol form of cyclopentanone is,
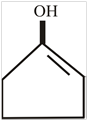
Explanation of Solution
The compound cyclopentanone contain a keto group. After the keto enol tautomerism the keto compound converted into an alcohol and a double bond. The structure of enol form of cyclopentanone is,

(e)
Interpretation: The structure of given compounds has to be drawn.:
The structure of enol form of cyclopentanone
Concept introduction: The
Carboxylate ion with a carbonyl group at the 3-position loses
(e)
Answer to Problem 48P
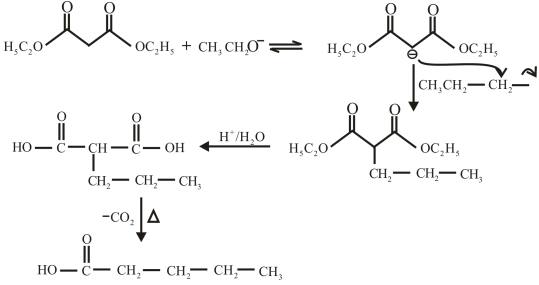
The structure of the carboxylic acid obtained from malonic ester synthesis by using propyl bromide is,
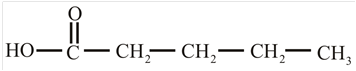
Explanation of Solution
The proton removed from the alpha carbon of malonic ester by the base. Then, there is a nucleophilic substitution reaction takes place between the propyl bromide and carbanion of malonic ester. The third step is acidic hydrolysis of an ester to form carboxylic acid. The fourth step is decarboxylation of the compound to form the desired product. The reaction and structure of given compound is,
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
