
CHEMISTRY:MOLECULAR..(LL)-PRINT..W/CODE
7th Edition
ISBN: 9781119457282
Author: JESPERSEN
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 2PE
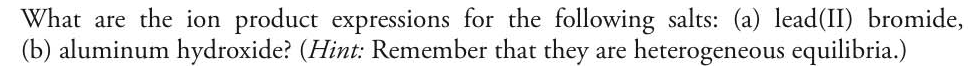
What are the ion product expressions for the following salts: (a) lead(II) bromide, (b) aluminum hydroxide? (Hint: Remember that they are heterogeneous equilibria.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10.
Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the
following compound. In order to recieve full credit, you MUST SHOW YOUR WORK!
H₂N
CI
OH
CI
カー
11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign
R/S configurations for all stereoisomers and indicate the relationship between each as
enantiomer, diastereomer, or meso.
NH2
H
HNH,
-18
b)
8.
Indicate whether the following carbocation rearrangements are likely to occur
Please explain your rational using 10 words or less
not likely to occur
• The double bond is still in the
Same position
+
Likely
to oc
occur
WHY?
-3
H3C
Brave
Chair Conformers. Draw the chair conformer of the following substituted
cyclohexane. Peform a RING FLIP and indicate the most stable
conformation and briefly explain why using 20 words or less.
CI
2
-cobs ??
MUST INDICATE H -2
-2
Br
EQ
Cl
OR
AT
Br
H&
most stable
WHY?
- 4
CH
12
Conformational Analysis. Draw all 6 conformers (one above each letter) of the
compound below looking down the indicated bond. Write the letter of the
conformer with the HIGHEST and LOWEST in energies on the lines provided.
NOTE: Conformer A MUST be the specific conformer of the structure as drawn below
-4 NOT
HOH
OH
3
Conformer A:
Br
OH
A
Samo
Br H
04
Br
H
H3
CH₂
H
anti
stagere
Br CH
clipsed
H
Brott
H
IV
H
MISSING 2
-2
B
C
D
E
F
X
6
Conformer with HIGHEST ENERGY:
13. (1
structure
LOWEST ENERGY:
Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the
corresponding to this name. HINT: Do not forget to indicate stereochemistry
when applicable.
a)
८८
2
"Br
{t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa
Parent name (noname)
4 Bromo
Sub = 2-methylethyl-4 Bromo nonane
b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane
# -2
-2
Chapter 17 Solutions
CHEMISTRY:MOLECULAR..(LL)-PRINT..W/CODE
Ch. 17 - What are the ion product expressions for the...Ch. 17 - What are the ion product expressions for the...Ch. 17 - The solubility of thallium(I) iodide, TlI, in...Ch. 17 - One liter of water will dissolve mol of ....Ch. 17 - Prob. 5PECh. 17 - Calculate the molar solubility of
Ch. 17 - Calculate the molar solubility of AgI in a...Ch. 17 - Prob. 8PECh. 17 - Calculate the ion product for the solution in...Ch. 17 - Calculate the ion product for a solution...
Ch. 17 - What precipitate might be expected if we mix ?...Ch. 17 - 50.0 mL of 0.10 M Pb(NO3)2 and 20.0 mL of 0.040 M...Ch. 17 - Determine whether adding an acid will increase the...Ch. 17 - Determine the molar solubility of Ag2CrO4in1.0MH+...Ch. 17 - What pH is needed to achieve a concentration of...Ch. 17 - What pH is needed to dissolve 2.00 g PbS in...Ch. 17 - If a solution contains calcium ions (0.25 M) and...Ch. 17 - Prob. 18PECh. 17 - Suppose a solution contains and is saturated with ...Ch. 17 - Consider a solution containing , both at...Ch. 17 - The Ksp for barium oxalate,...Ch. 17 - A solution contains calcium nitrate and nickel...Ch. 17 - Calculate the solubility of silver chloride in ...Ch. 17 - How many moles of have to be added to 1.0 L of...Ch. 17 - Equilibria in Solutions of Slightly Soluble Salts...Ch. 17 - Equilibria in Solutions of Slightly Soluble Salts...Ch. 17 - Equilibria in Solutions of Slightly Soluble Salts...Ch. 17 - Equilibria in Solutions of Slightly Soluble...Ch. 17 - Prob. 5RQCh. 17 - Prob. 6RQCh. 17 - Equilibria in Solutions of Slightly Soluble Salts...Ch. 17 - Solubility of Basic Salts Is Influenced by...Ch. 17 - Prob. 9RQCh. 17 - Solubility of Basic Salts Is Influenced by...Ch. 17 - Solubility of Basic Salts Is Influenced by Acids...Ch. 17 - Solubility of Basic Salts Is Influenced by Acids A...Ch. 17 - Solubility of Basic Salts Is Influenced by Acids...Ch. 17 - Solubility of Basic Salts Is Influenced by Acids...Ch. 17 - Equilibria in Solutions of Metal Oxides and...Ch. 17 - Equilibria in Solutions of Metal Oxides and...Ch. 17 - Equilibria in Solutions of Metal Oxides and...Ch. 17 - Selective Precipitation Use Le Chatelier's...Ch. 17 - Prob. 19RQCh. 17 - Selective Precipitation If you had a solution with...Ch. 17 - Equilibria Involving Complex Ions Explain the...Ch. 17 - Equilibria Involving Complex Ions What is a...Ch. 17 - Complexation and Solubility
17.23 Using Le...Ch. 17 - Complexation and Solubility For...Ch. 17 - Prob. 25RQCh. 17 - Equilibria in Solutions of Slightly Soluble...Ch. 17 - Prob. 27RQCh. 17 - Prob. 28RQCh. 17 - Prob. 29RQCh. 17 - Prob. 30RQCh. 17 - Prob. 31RQCh. 17 - Which compound is more soluble in water,...Ch. 17 - 17.33 In water, the solubility of lead(II)...Ch. 17 - The solubility of zinc oxalate is 7.9103M....Ch. 17 - 17.35 Barium sulfate is so insoluble that it can...Ch. 17 - A student found that a maximum of 0.800 g silver...Ch. 17 - Prob. 37RQCh. 17 - A student prepared a saturated solution of CaCrO4...Ch. 17 - At 25C, the molar solubility of silver phosphate...Ch. 17 - 17.40 The molar solubility of barium phosphate in...Ch. 17 - What is the molar solubility of PbBr2 in water?Ch. 17 - 17.42 What is the molar solubility of in water?
Ch. 17 - Calculate the molar solubility of Zn(CN)2 in...Ch. 17 - Calculate the molar solubility of PbF2 in water....Ch. 17 - 17.45 At , the value of for , and that for ....Ch. 17 - At 25C, the value of Ksp for AgCNis6.010-17 and...Ch. 17 - A salt whose formula is MX has a Ksp equal to...Ch. 17 - 17.48 A salt having a formula of the type has ....Ch. 17 - Calcium sulfate is found in plaster. At 25C the...Ch. 17 - Chalk is CaCO3, and at 25CitsKsp=3.410 What is the...Ch. 17 - It was found that the molar solubility of BaSO3 in...Ch. 17 - 17.52 The molar solubility of . What is the value...Ch. 17 - Prob. 53RQCh. 17 - Mercury(I) chloride has Ksp=1.41018. Calculate the...Ch. 17 - Calculate the molar solubility of Mg(OH)2 in a...Ch. 17 - 17.56 Calculate the molar solubility of in a...Ch. 17 - 17.57 What is the highest concentration of that...Ch. 17 - 17.58 Will lead (II) bromide be less soluble in...Ch. 17 - Calculate the molar solubility of...Ch. 17 - 17.60 What is the molar solubility of in (a) 0.20...Ch. 17 - 17.61 What is the molar solubility of in a buffer...Ch. 17 - What is the molar solubility of Ca(OH)2 in (a)...Ch. 17 - In an experiment, 2.20gofNaOH(s) is added to 250...Ch. 17 - Suppose that 1.75 g of NaOH(s) is added to 250 mL...Ch. 17 - 17.65 Docs a precipitate of form when are...Ch. 17 - Prob. 66RQCh. 17 - 17.67 Docs a precipitate of form if 50.0 ml. of ...Ch. 17 - Prob. 68RQCh. 17 - 17.69 Suppose that 50.0 mL each of 0.0100 M...Ch. 17 - If a solution of 0.10 M Mn2+ and 0.10 M Cd2+ is...Ch. 17 - In an aqueous suspension of Ca(OH)2, the only...Ch. 17 - Suppose that 25.0 mL of 0.10MHCl is added to 1.000...Ch. 17 - *17.73 When solid is added to a suspension of ...Ch. 17 - *17.74 After solid was added to a slightly basic...Ch. 17 - *17.75 Will a precipitate form in a solution made...Ch. 17 - 17.76 What is the molar solubility of in water?...Ch. 17 - Solubility of Basic Salts Is Influenced by...Ch. 17 - Solubility of Basic Salts Is Influenced by...Ch. 17 - *17.79 What is the molar solubility of in pure...Ch. 17 - 17.80 What is the molar solubility of in pure...Ch. 17 - How many grams of solid sodium acetate must be...Ch. 17 - How many grams of solid potassium fluoride must be...Ch. 17 - What is the molar solubility of Mg(OH)2 in 0.1MNH3...Ch. 17 - *17.84 If 100 mL of is added to 0.400 L of a...Ch. 17 - Equilibria in Solutions of Metal Oxides and...Ch. 17 - Prob. 86RQCh. 17 - *17.87 Does nickel(II) sulfide dissolve in 4 M...Ch. 17 - Does iron(II) sulfide dissolve in 8 M HCl? Perform...Ch. 17 - Selective Precipitation Both AgCl and AgI are very...Ch. 17 - Prob. 90RQCh. 17 - 17.91 What value of and what pH permit the...Ch. 17 - What pH would yield the maximum separation of Mn2+...Ch. 17 - Prob. 93RQCh. 17 - Kidney stones often contain insoluble calcium...Ch. 17 - 17.95 Solid is added to a solution of . After...Ch. 17 - What value of [ H+ ] and what pH would allow the...Ch. 17 - Prob. 97RQCh. 17 - *17.98 In the metal plating industry, the waste is...Ch. 17 - Equilibria Involving Complex Ions
17.99 Write the...Ch. 17 - Equilibria Involving Complex Ions
17.100 Write the...Ch. 17 - Prob. 101RQCh. 17 - Prob. 102RQCh. 17 - Prob. 103RQCh. 17 - Prob. 104RQCh. 17 - For PbCl3, Kform=2.510 . Use this information plus...Ch. 17 - The overall formation constant for Ag(CN)2 equals...Ch. 17 - How many grams of solid NaCN have to be added to...Ch. 17 - Prob. 108RQCh. 17 - Silver iodide is very insoluble and can be...Ch. 17 - 17.110 Silver forms a sparingly soluble iodide...Ch. 17 - The formation constant for Ag(CN)2-equals5.341018....Ch. 17 - 17.112 Suppose that some dipositive cation, , is...Ch. 17 - (Calculate the molar solubility of...Ch. 17 - The molar solubility of Zn(OH)2in1.0MNH3 is...Ch. 17 - What ratio of Ksp values will allow for the use of...Ch. 17 - A student had a solution that contained...Ch. 17 - Suppose that 50.0 mL of 0.12 M AgNO3 is added to...Ch. 17 - Prob. 118RQCh. 17 - Prob. 119RQCh. 17 - In Example 17.11 we say, there are relatively few...Ch. 17 - *17.121 What is the molar solubility of ? What is...Ch. 17 - *17.122 An old method for mining gold was to wash...Ch. 17 - What is the molar concentration of Cu2+ ion in a...Ch. 17 - On the basis of the KspofAl(OH)3, what would be...Ch. 17 - The compound EDTA is used to remove soap scum, as...Ch. 17 - A chemist has a solution that contains a mixture...Ch. 17 - You are given a sample containing , both with...Ch. 17 - Prob. 128RQCh. 17 - In modern construction, walls and ceilings are...Ch. 17 - *17.130 How many milliliters of would have to be...Ch. 17 - Marble is composed primarily of CaCO3, a slightly...Ch. 17 - *17.132 The pH of a saturated solution of is 9.8....Ch. 17 - The osmotic pressure of a saturated solution of...Ch. 17 - A saturated solution of PbCl2 has a freezing point...Ch. 17 - 17.135 Consider mercury(II) sulfide, , which has a...Ch. 17 - If aqueous ammonia is added gradually to a...Ch. 17 - From a practical standpoint, can you effectively...Ch. 17 - 17.139 In older textbooks, the solubility...Ch. 17 - Suppose two silver wires, one coated with silver...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Problem 35 through 40 show a free-body diagram. For each:
Identify the direction of the acceleration vector and...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Write the Lewis structure for each ionic compound. a. NaF b. CaO c. SrBr2 d. K2O
Introductory Chemistry (6th Edition)
1. How many cervical, thoracic, lumbar, sacral, and coccygeal vertebrae are normally present in the vertebral ...
Human Anatomy & Physiology (2nd Edition)
MAKE CONNECTIONS Using what you know of gene expression in a cell, explain what causes the traits of parents (...
Campbell Biology (11th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
Answer the following questions for each compound: a. How many signals are in its 13C NMR spectrum? b. Which sig...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY