
What would be the effect of carrying out the sodium iodide in acetone reaction with the
Interpretation: The effect of carrying out the sodium iodide in acetone reaction with the alkyl halide using iodide solution half as concentrated is to be interpreted.
Concept introduction: The reaction of alkyl halide with sodium iodide results the formation of alkyl iodide. The reaction occurs in the presence of acetone as the solvent. It is named as Finkelstein reaction and is followed by
Answer to Problem 1Q
In the presence of half concentrated iodide solution, the
Explanation of Solution
The mechanism of the given reaction can be shown as given below:
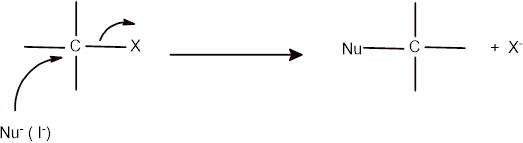
Here the iodide ions act as nucleophile and involve in the rate determining step as reaction follows the
Therefore, with decrease in the concentration of iodide ion, the rate of reaction will also halves that yield only half of the product.
In
Want to see more full solutions like this?
Chapter 17 Solutions
Macroscale and Microscale Organic Experiments
- Complete the reaction in the fewest number of steps as possible, Draw all intermediates (In the same form as the picture provided) and provide all reagents.arrow_forwardPlease provide steps to work for complete understanding.arrow_forwardPlease provide steps to work for complete understanding.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardA certain chemical reaction releases 24.7 kJ/g of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce 1460. J of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. mass M 0.0 x μ 00 1 Garrow_forwardPlease don't used hand raiting and don't used Ai solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



