
Concept explainers
Diagram the following galvanic cell, indicating the directionof flow of electrons in the external circuit and themotion of ions in the salt bridge.
Write a balanced equation for the overall reaction in thiscell.
Interpretation:
The given galvanic cell needs to be drawn and a balanced equation for the overall reaction in the cell is to be determined.
Concept introduction:
A galvanic cell is also known as a voltaic cell. It is an electrochemical cell where oxidation-reduction reactions take place and result in the generation of an electric current. In a simple galvanic cell, there are two electrodes: cathode and anode. Each of the electrodes is immersed in their respective metal ions solutions. Thus, there are two compartments of the cell. Both compartments of the cell are connected by a salt bridge which helps in the movement of ions between the compartments of the cell.
Answer to Problem 1P
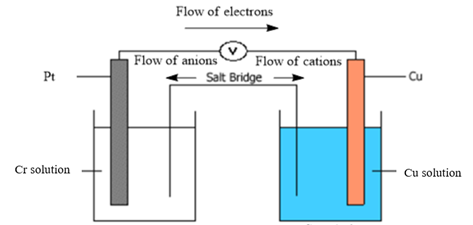
Diagram of galvanic cell:
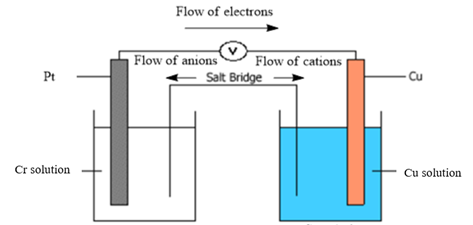
Balanced overall cell reaction:
Explanation of Solution
The given cell notation is as follows:
In a cell notation, oxidation is written always before reduction. Thus, in the given case,
Here, platinum is an inert electrode that is the source or sink for electrons. It does not play any chemical role in the electrode reaction.
Since oxidation takes place at the anode and reduction at the cathode, the anodic and cathodic reactions (half-reactions) of the cell can be represented as follows:
At the anode, oxidation takes place as follows:
At the cathode, reduction takes place as follows:
From the above two half-reactions, the overall cell reaction can be obtained as follows:
Thus, the balanced overall cell reaction is as follows:
Now, in a typical galvanic cell, the flow of anions (negatively charged ions) takes place from cathode to anode, and the flow of cations (positively charged ions) takes place from anode to cathode through a salt bridge. Also, in the external circuit, the movement of electrons takes place from anode to cathode. The diagram of the given galvanic cell can be represented as follows:
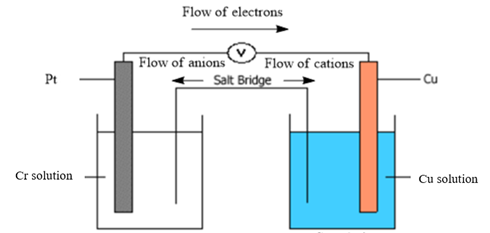
Thus, the diagram of the galvanic cell and the balanced overall cell reaction is as follows:
Want to see more full solutions like this?
Chapter 17 Solutions
Student Solutions Manual for Oxtoby/Gillis/Butler's Principles of Modern Chemistry, 8th
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
- Given the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forwardMatch each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





