
Chemistry (OER)
19th Edition
ISBN: 9781947172623
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 19E
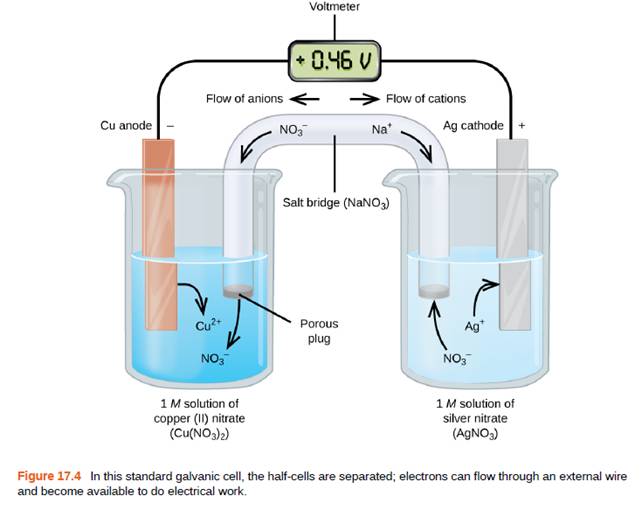
Why is a salt bridge necessary in galvanic cells like the one in Figure 17.4?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the proper IUPAC name only for the following
compound. Dashes, commas, and spaces must be used
correctly, but do not use italics in Canvas.
The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 M
What is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)
Chapter 17 Solutions
Chemistry (OER)
Ch. 17 - If a 2.5 A current is run through a circuit for 35...Ch. 17 - For the scenario in the previous question, how...Ch. 17 - For each of the following balanced half-reactions,...Ch. 17 - For each of the following balanced half-reactions,...Ch. 17 - Given the following pairs of balanced...Ch. 17 - Balance the following in acidic solution: (a)...Ch. 17 - Identify the species that undergoes oxidation, the...Ch. 17 - Balance the following in acidic solution: (a)...Ch. 17 - Identify the species that was oxidized, the...Ch. 17 - Why is it not possible for hydroxide ion (OH-) to...
Ch. 17 - Why is it not possible for hydrogen ion (H+) to...Ch. 17 - Why must the charge balance in oxidation-reduction...Ch. 17 - Write the following balanced reactions using cell...Ch. 17 - Given the following cell notations, determine the...Ch. 17 - For the cell notations in the previous problem,...Ch. 17 - Balance the following reactions and write the...Ch. 17 - Identify the species oxidized species reduced, and...Ch. 17 - From the information provided, use cell notation...Ch. 17 - Why is a salt bridge necessary in galvanic cells...Ch. 17 - An active (metal) electrode was found to gain mass...Ch. 17 - An active (metal) electrode was found to lose mass...Ch. 17 - The mass of three different metal electrodes, each...Ch. 17 - For each reaction listed, determine its standard...Ch. 17 - For each reaction listed, determine its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - For the standard cell potentials given here,...Ch. 17 - For the ?G values given here, determine the...Ch. 17 - Determine the standard cell potential and the cell...Ch. 17 - Determine G and G for each of the reactions in...Ch. 17 - Use the data in Appendix L to determine the...Ch. 17 - What are the desirable qualities of an electric...Ch. 17 - List some things that are typically considered...Ch. 17 - Consider a battery made from one half-cell that...Ch. 17 - Consider a battery with the overall reaction:...Ch. 17 - An inventor proposes using a SHE (standard...Ch. 17 - Why do batteries go dead, but fuel cells do not?Ch. 17 - Explain what happens to battery voltage as a...Ch. 17 - Using the information thus far in this chapter,...Ch. 17 - Which member of each pair of metals is more likely...Ch. 17 - Consider the following metals: Ag, Au, Mg, Ni, and...Ch. 17 - Aluminum (E Al 3+/Al=2.07V) is more easily...Ch. 17 - If a sample of iron and a sample of zinc come into...Ch. 17 - Suppose you have three different metals. A, B, and...Ch. 17 - Why would a sacrificial anode made of lithium...Ch. 17 - Identify the reaction at the anode, reaction at...Ch. 17 - What mass of each product is produced in each of...Ch. 17 - How long would it take to reduce 1 mole of each of...Ch. 17 - A current of 2.345 A passes through the cell shown...Ch. 17 - An irregularly shaped metal part made from a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
According to the logistic growth equation dNdt=rN(KN)K (A) the number of individuals added per unit time is gre...
Campbell Biology (11th Edition)
1. What are the main organs of the skeletal system?
Human Anatomy & Physiology (2nd Edition)
In your own words, briefly distinguish between relative dates and numerical dates.
Applications and Investigations in Earth Science (9th Edition)
1. A person gets in an elevator on the ground floor and rides it to the top floor of a building. Sketch a veloc...
College Physics: A Strategic Approach (3rd Edition)
Choose the more metallic element from each pair. a. Sb or Pb b. K or Ge c. Ge or Sb d. As or Sn
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardWhat is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forward
- Provide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forwardQuestion 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forwardPlease assign all the carbons for C-NMR and hydrogen for H-NMR. Please if I can get that less than hourarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY