
Interpretation:
The
Concept Introduction:
Carboxylic acid: One
Amide: One
Amide Formation: Amide is formed when a carboxylic acid reacts with an amine or ammonia.
- Primary amide is produce when a carboxylic acid reacts with ammonia.
- Secondary and tertiary amide is produce when a carboxylic acid reacts with primary and secondary amine respectively.
- Ammonium salt is formed when tertiary amine and a carboxylic acid reacts forming an ionic compound with a carboxylate acid anion and a trialkyl ammonium cation since there is no hydrogen atom in trialkyl amine
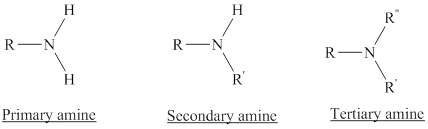
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Aromatic Compounds: Compounds that are planar, cyclic and having
Trending nowThis is a popular solution!

Chapter 17 Solutions
EP FUND.OF GENERAL,ORG...-MOD.MASTERING
- Draw the reaction between sphingosine and arachidonic acid. Draw out the full structures.arrow_forwardDraw both cis and trans oleic acid. Explain why cis-oleic acid has a melting point of 13.4°C and trans-oleic acid has a melting point of 44.5°C.arrow_forwardDraw the full structure of the mixed triacylglycerol formed by the reaction of glycerol and the fatty acids arachidic, lauric and trans-palmitoleic. Draw the line structure.arrow_forward
- Draw out the structure for lycopene and label each isoprene unit. "Where is lycopene found in nature and what health benefits does it provide?arrow_forwardWhat does it mean to be an essential fatty acid? What are the essential fatty acids?arrow_forwardCompare and contrast primary and secondary active transport mechanisms in terms of energy utilisation and efficiency. Provide examples of each and discuss their physiological significance in maintaining ionic balance and nutrient uptake. Rubric Understanding the key concepts (clearly and accurately explains primary and secondary active transport mechanisms, showing a deep understanding of their roles) Energy utilisation analysis ( thoroughly compares energy utilisation in primary and secondary transport with specific and relevant examples Efficiency discussion Use of examples (provides relevant and accurate examples (e.g sodium potassium pump, SGLT1) with clear links to physiological significance. Clarity and structure (presents ideas logically and cohesively with clear organisation and smooth transition between sections)arrow_forward
- 9. Which one of the compounds below is the major organic product obtained from the following reaction sequence, starting with ethyl acetoacetate? 요요. 1. NaOCH2CH3 CH3CH2OH 1. NaOH, H₂O 2. H3O+ 3. A OCH2CH3 2. ethyl acetoacetate ii A 3. H3O+ OH B C D Earrow_forward7. Only one of the following ketones cannot be made via an acetoacetic ester synthesis. Which one is it? Ph کہ A B C D Earrow_forward2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forward
- what is a protein that contains a b-sheet and how does the secondary structure contributes to the overall function of the protein.arrow_forwarddraw and annotate a b-sheet and lable the hydrogen bonding. what is an example that contains the b-sheet and how the secondary structure contributes to the overall function of your example protein.arrow_forwardFour distinct classes of interactions (inter and intramolecular forces) contribute to a protein's tertiary and quaternary structures. Name the interaction then describe the amino acids that can form this type of interaction. Draw and annotate a diagram of the interaction between two amino acids.arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning





