
Concept explainers
(a)
Interpretation:
The synthesis of the given
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is shown below:
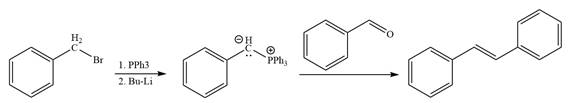
Explanation of Solution
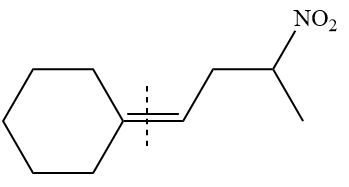
The given structure of alkene is
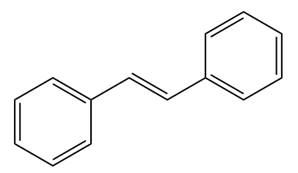
In the above structure, the
By undoing a Wittig reaction in retrosynthetic analysis,
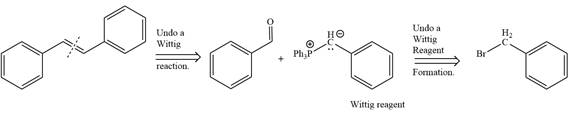
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehydewould appears as follows:
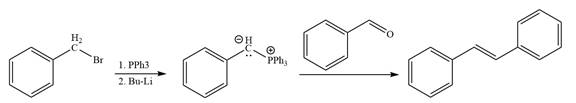
The synthesis of given alkene from an alkyl halide and a ketone or aldehyde is shown.
(b)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
Method 2:
Explanation of Solution
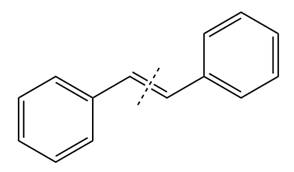
The given structure of alkene is
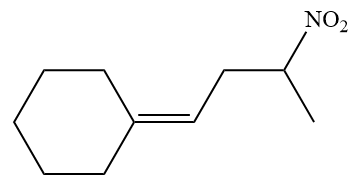
In the above structure, the
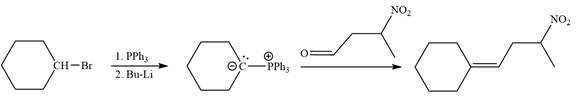
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
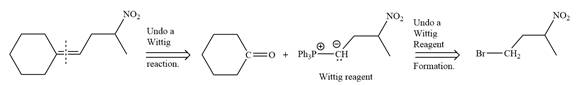
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:
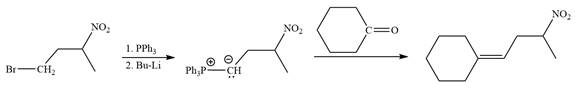
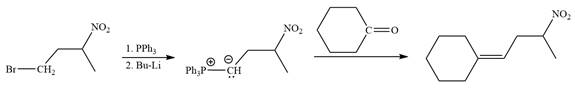
Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
In the forward direction, the synthesis of the given alkene from an alkyl halide and an aldehyde would appear as follows:

The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
(c)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
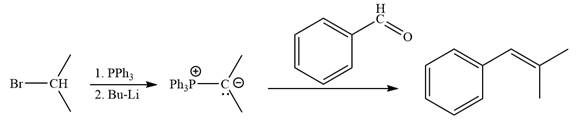
Method 2:
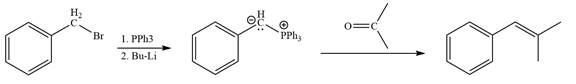
Explanation of Solution
The given structure of alkene is
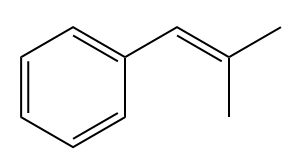
In the above structure, the
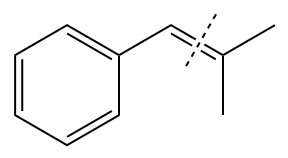
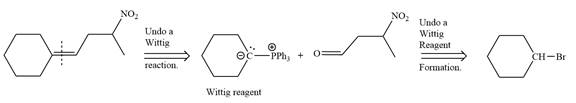
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
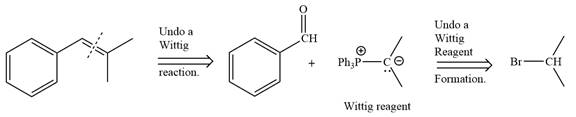
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehyde would appear as follows:

Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
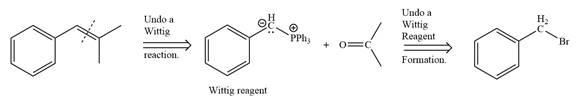
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:

The synthesis of the given unsymmetric alkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Draw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forwardBookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forward
- Predict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forwardif the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
