
Concept explainers
Propose a structure consistent with each set of data.
a. Compound A:
b. Compound B:
(a)
Interpretation: A structure consistent with the given set of data is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 17.56P
A structure consistent with the given set of data is shown below.
Explanation of Solution
The given sets of
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
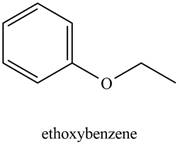
The possible structure of the Compound A, based on the above analysis is,
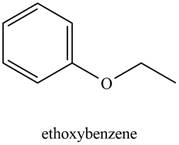
Figure 1
A structure consistent with the given set of data is shown in Figure 1.
(b)
Interpretation: A structure consistent with the given set of data is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 17.56P
A structure consistent with the given set of data is shown below.
Explanation of Solution
The given sets of
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift values at
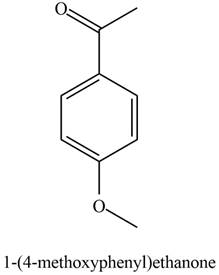
The possible structure of the Compound B, based on the above analysis is,
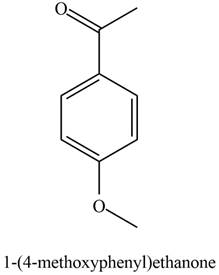
Figure 2
A structure consistent with the given set of data is shown in Figure 2.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry-Package(Custom)
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

